JEE Advance - Chemistry (2022 - Paper 1 Online)
$2 \mathrm{~mol} \,\mathrm{of}\, \mathrm{Hg}(\mathrm{g})$ is combusted in a fixed volume bomb calorimeter with excess of $\mathrm{O}_{2}$ at $298 \mathrm{~K}$ and 1 atm into $\mathrm{HgO}(s)$. During the reaction, temperature increases from $298.0 \mathrm{~K}$ to $312.8 \mathrm{~K}$. If heat capacity of the bomb calorimeter and enthalpy of formation of $\mathrm{Hg}(g)$ are $20.00 \mathrm{~kJ} \mathrm{~K}^{-1}$ and $61.32 \mathrm{~kJ}$ $\mathrm{mol}^{-1}$ at $298 \mathrm{~K}$, respectively, the calculated standard molar enthalpy of formation of $\mathrm{HgO}(s)$ at 298 $\mathrm{K}$ is $\mathrm{X}\, \mathrm{kJ}\, \mathrm{mol}^{-1}$. The value of $|\mathrm{X}|$ is _________ .
[Given: Gas constant $\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ ]
The reduction potential $\left(E^{0}\right.$, in $\left.\mathrm{V}\right)$ of $\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})$ is __________.
[Given: $E_{\left(\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{MnO}_{2}(\mathrm{~s})\right)}^{0}=1.68 \mathrm{~V} ; E_{\left(\mathrm{MnO}_{2}(\mathrm{~s}) / \mathrm{Mn}^{2+}(\mathrm{aq})\right)}^{0}=1.21 \mathrm{~V} ; E_{\left(\mathrm{Mn}^{2+}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})\right)}^{0}=-1.03 \mathrm{~V}$ ]
A solution is prepared by mixing $0.01 \mathrm{~mol}$ each of $\mathrm{H}_{2} \mathrm{CO}_{3}, \mathrm{NaHCO}_{3}, \mathrm{Na}_{2} \mathrm{CO}_{3}$, and $\mathrm{NaOH}$ in $100 \mathrm{~mL}$ of water. $p \mathrm{H}$ of the resulting solution is _________.
[Given: $p \mathrm{~K}_{\mathrm{a} 1}$ and $p \mathrm{~K}_{\mathrm{a} 2}$ of $\mathrm{H}_{2} \mathrm{CO}_{3}$ are $6.37$ and 10.32, respectively; $\log 2=0.30$ ]
The treatment of an aqueous solution of $3.74 \mathrm{~g}$ of $\mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}$ with excess KI results in a brown solution along with the formation of a precipitate. Passing $\mathrm{H}_{2} \mathrm{~S}$ through this brown solution gives another precipitate $\mathbf{X}$. The amount of $\mathbf{X}$ (in $g$ ) is ___________.
[Given: Atomic mass of $\mathrm{H}=1, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{~S}=32, \mathrm{~K}=39, \mathrm{Cu}=63, \mathrm{I}=127$ ]
Dissolving $1.24 \mathrm{~g}$ of white phosphorous in boiling $\mathrm{NaOH}$ solution in an inert atmosphere gives a gas $\mathbf{Q}$. The amount of $\mathrm{CuSO}_{4}$ (in g) required to completely consume the gas $\mathbf{Q}$ is _________.
[Given: Atomic mass of $\mathrm{H}=1, \mathrm{O}=16, \mathrm{Na}=23, \mathrm{P}=31, \mathrm{~S}=32, \mathrm{Cu}=63$ ]
Consider the following reaction.

On estimation of bromine in $1.00 \mathrm{~g}$ of $\mathbf{R}$ using Carius method, the amount of $\mathrm{AgBr}$ formed (in $\mathrm{g}$ ) is ___________.
[Given: Atomic mass of $\mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{P}=31, \mathrm{Br}=80, \mathrm{Ag}=108]$
If the reaction sequence given below is carried out with 15 moles of acetylene, the amount of the product $\mathbf{D}$ formed (in $\mathrm{g}$ ) is ___________ .
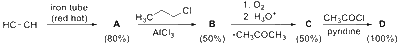
The yields of $\mathbf{A}, \mathbf{B}, \mathbf{C}$ and $\mathbf{D}$ are given in parentheses.
[Given: Atomic mass of $\mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Cl}=35$ ]
Match the rate expressions in LIST-I for the decomposition of $X$ with the corresponding profiles provided in LIST-II. $X_{\mathrm{s}}$ and $\mathrm{k}$ are constants having appropriate units.
| List-I | List-II |
|---|---|
| (I) rate $=\frac{\mathrm{k}[\mathrm{X}]}{\mathrm{X}_{\mathrm{s}}+[\mathrm{X}]}$ under all possible initial concentrations of $\mathrm{X}$ |
(P)  |
| (II) rate $=\frac{k[X]}{X_{s}+[X]}$ where initial concentrations of $X$ are much less than $X_{s}$ |
(Q) 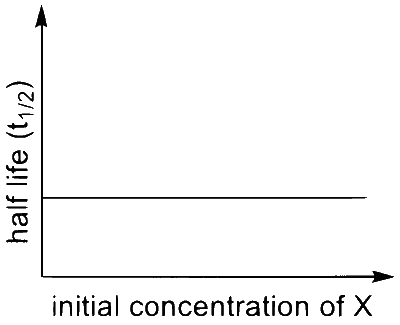 |
| (III) rate $=\frac{k[X]}{X_{s}+[X]}$ where initial concentrations of $\mathrm{X}$ are much higher than $X_{s}$ |
(R) 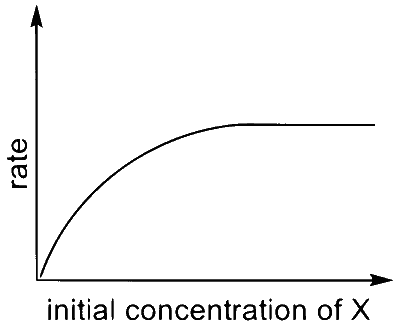 |
| (IV) rate $=\frac{k[X]^{2}}{X_{s}+[X]}$ where initial concentration of $X$ is much higher than $\mathrm{X}_{\mathrm{s}}$ |
(S) 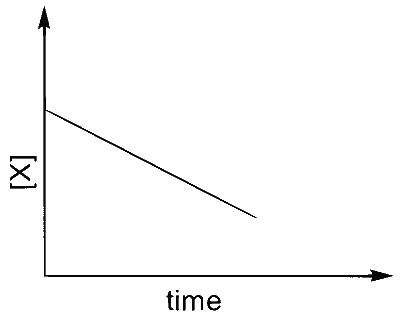 |
(T) 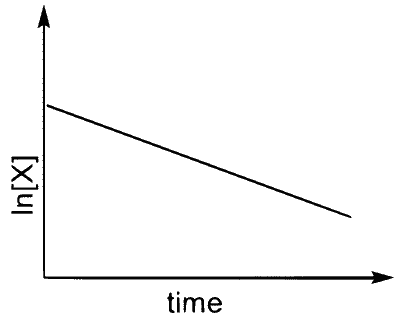 |
LIST-I contains compounds and LIST-II contains reactions
| List-I | List-II |
|---|---|
| (I) $\mathrm{H}_{2} \mathrm{O}_{2}$ |
(P) $\mathrm{Mg}\left(\mathrm{HCO}_{3}\right)_{2}+\mathrm{Ca}(\mathrm{OH})_{2} \rightarrow$ |
| (II) $\mathrm{Mg}(\mathrm{OH})_{2}$ |
(Q) $\mathrm{BaO}_{2}+\mathrm{H}_{2} \mathrm{SO}_{4} \rightarrow$ |
| (III) $\mathrm{BaCl}_{2}$ |
(R) $\mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{MgCl}_{2} \rightarrow$ |
| (IV) $\mathrm{CaCO}_{3}$ |
(S) $\mathrm{BaO}_{2}+\mathrm{HCl} \rightarrow$ |
| (T) $\mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}+\mathrm{Ca}(\mathrm{OH})_{2} \rightarrow$ |
Match each compound in LIST-I with its formation reaction(s) in LIST-II, and choose the correct option
LIST-I contains metal species and LIST-II contains their properties.
| List-I | List-II |
|---|---|
| (I) $\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{4-}$ |
(P) $t_{2 \mathrm{g}}$ orbitals contain 4 electrons |
| (II) $\left[\mathrm{RuCl}_{6}\right]^{2-}$ | (Q) $\mu$ (spin-only $)=4.9 \mathrm{BM}$ |
| (III) $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ |
(R) low spin complex ion |
| (IV) $\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ |
(S) metal ion in $4+$ oxidation state |
| (T) $d^{4}$ species |
[Given: Atomic number of $\mathrm{Cr}=24, \mathrm{Ru}=44, \mathrm{Fe}=26$ ]
Match each metal species in LIST-I with their properties in LIST-II, and choose the correct option
Match the compounds in LIST-I with the observations in LIST-II, and choose the correct option.
| List-I | List-II |
|---|---|
| (I) Aniline |
(P) Sodium fusion extract of the compound on boiling with $\mathrm{FeSO}_{4}$, followed by acidification with conc. $\mathrm{H}_{2} \mathrm{SO}_{4}$, gives Prussian blue color. |
| (II) $o$-Cresol | (Q) Sodium fusion extract of the compound on treatment with sodium nitroprusside gives blood red color. |
| (III) Cysteine |
(R) Addition of the compound to a saturated solution of $\mathrm{NaHCO}_{3}$ results in effervescence. |
| (IV) Caprolactam |
(S) The compound reacts with bromine water to give a white precipitate. |
| (T) Treating the compound with neutral $\mathrm{FeCl}_{3}$ solution produces violet color. |




