JEE Advance - Chemistry (2022 - Paper 1 Online - No. 8)
If the reaction sequence given below is carried out with 15 moles of acetylene, the amount of the product $\mathbf{D}$ formed (in $\mathrm{g}$ ) is ___________ .
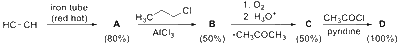
The yields of $\mathbf{A}, \mathbf{B}, \mathbf{C}$ and $\mathbf{D}$ are given in parentheses.
[Given: Atomic mass of $\mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Cl}=35$ ]
Answer
136
136g
- OR
Explanation
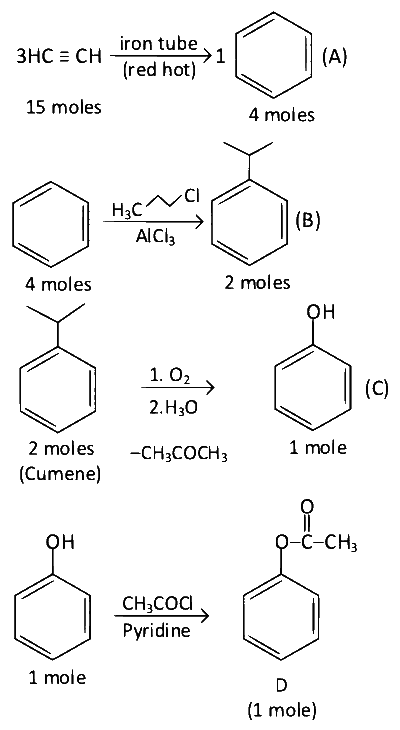
Molecular formula of D is C8H8O2
Molar mass of D is ($$12\times8+8\times1+16\times2$$) = 136 g
$$\therefore$$ Mass of D is 136
Comments (0)


