JEE Advance - Chemistry (2022 - Paper 1 Online - No. 9)
For diatomic molecules, the correct statement(s) about the molecular orbitals formed by the overlap of two $2 p_{z}$ orbitals is(are)
$\sigma$ orbital has a total of two nodal planes.
$\sigma^{*}$ orbital has one node in the $x z$-plane containing the molecular axis.
$\pi$ orbital has one node in the plane which is perpendicular to the molecular axis and goes through the center of the molecule.
$\pi^{*}$ orbital has one node in the $x y$-plane containing the molecular axis.
Explanation
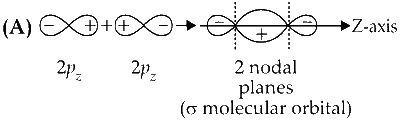
Option (A) is correct.
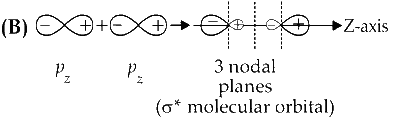
Option (B) is incorrect.
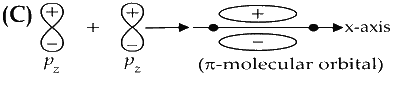
$\pi^*$ orbital has zero node is the plane which is perpendicular to the molecular axis and goes through the center of molecule.
So, option (C) is incorrect.
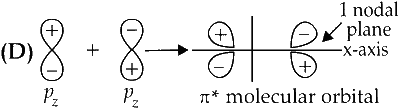
$\pi^*$ Orbital has one node in the $x y-$ plane containing the molecular axis.
Option (D) is correct.
Comments (0)


