JEE Advance - Chemistry (2022 - Paper 1 Online - No. 15)
Match the rate expressions in LIST-I for the decomposition of $X$ with the corresponding profiles provided in LIST-II. $X_{\mathrm{s}}$ and $\mathrm{k}$ are constants having appropriate units.
| List-I | List-II |
|---|---|
| (I) rate $=\frac{\mathrm{k}[\mathrm{X}]}{\mathrm{X}_{\mathrm{s}}+[\mathrm{X}]}$ under all possible initial concentrations of $\mathrm{X}$ |
(P)  |
| (II) rate $=\frac{k[X]}{X_{s}+[X]}$ where initial concentrations of $X$ are much less than $X_{s}$ |
(Q) 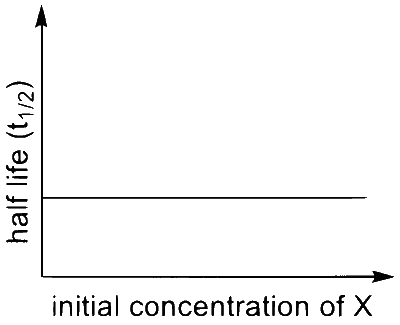 |
| (III) rate $=\frac{k[X]}{X_{s}+[X]}$ where initial concentrations of $\mathrm{X}$ are much higher than $X_{s}$ |
(R) 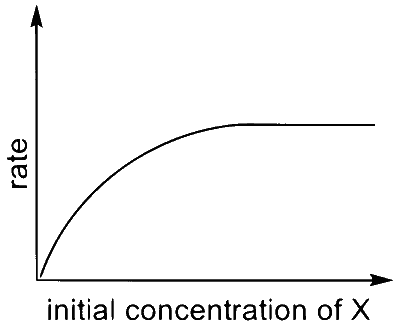 |
| (IV) rate $=\frac{k[X]^{2}}{X_{s}+[X]}$ where initial concentration of $X$ is much higher than $\mathrm{X}_{\mathrm{s}}$ |
(S) 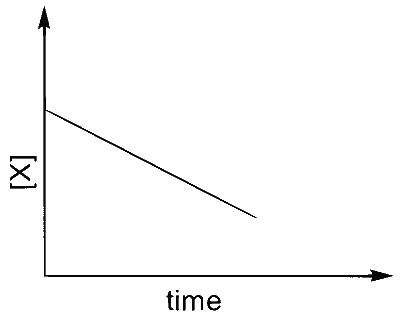 |
(T) 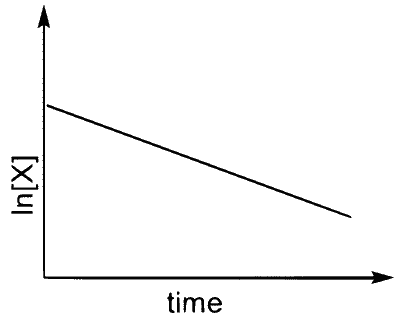 |
Explanation
rate = $$\frac{k[x]}{X_s+[X]}$$
Case-1 : $$[X] > > {X_s};[X] + {X_s} \approx [X]$$
rate $$ = {{k[X]} \over {[X]}} = k$$ (Zero order w.r.t. X)
I $$\to$$ P, S
Case 2 : $$[X] < < {X_s};[X] + {X_s} \approx {X_s}$$
$$\therefore$$ rate $$ = {{k[X]} \over {{X_s}}} = k'[X]$$ (1st order w.r.t. X)
$$\therefore$$ I $$\to$$ Q, T
Case-3 : $$[X] \approx {X_s}$$
rate $$ = {{k[X]} \over {{X_s} + [X]}}$$
In this case curve-R given in List-II will match.
$$\therefore$$ I $$\to$$ P, Q, R, S, T (The graph of half-life should start from origin)
(II) rate $$ = {{k[X]} \over {{X_s} + [X]}}$$
$$\because$$ $$[X] < < {X_s}$$
$$\therefore$$ $${X_s} + [X] \approx {X_s}$$
$$\therefore$$ Rate $$ = {{k[X]} \over {{X_s}}} = k'[X]$$ (1st order w.r.t. X)
$$\therefore$$ II $$\to$$ Q, T
(III) rate $$ = {{k[X]} \over {{X_s} + [X]}}$$
$$\because$$ $$[X] > > {X_s}$$
$$\therefore$$ $${X_s} + [X] \approx [X]$$
$$\therefore$$ rate $$ = {{k[X]} \over {[X]}} = k$$ (Zero order w.r.t. X)
$$\therefore$$ III $$\to$$ P, S
(IV) rate $$ = {{k{{[X]}^2}} \over {{X_s} + [X]}}$$
$$\because$$ $$[X] > > {X_s}$$
$$\therefore$$ $${X_s} + [X] \approx [X]$$
$$\therefore$$ rate $$ = {{k{{[X]}^2}} \over {[X]}} = k[X]$$ (1st order w.r.t. X)
$$\therefore$$ IV $$\to$$ Q, T
Comments (0)


