JEE Advance - Chemistry (2022 - Paper 1 Online - No. 14)
Considering the following reaction sequence,

the correct option(s) is(are)
$\mathbf{P}=\mathrm{H}_{2} / \mathrm{Pd}$, ethanol
$\mathbf{R}=\mathrm{NaNO}_{2} / \mathrm{HCl}$
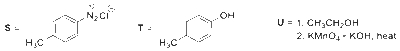
$\mathbf{U}=1 . \mathrm{H}_{3} \mathrm{PO}_{2}$
2. $\mathrm{KMnO}_{4}-\mathrm{KOH}$, heat
2. $\mathrm{KMnO}_{4}-\mathrm{KOH}$, heat
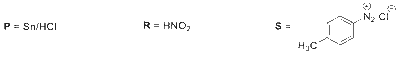

Explanation
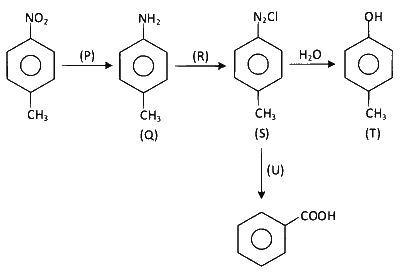
$$\to$$ P may be $$\to$$ H2/Pd, ethanol; Sn/HCl
$$\to$$ R may be $$\to$$ NaNO2/HCl; HNO2
$$\to$$ U may be $$\to$$ (i) H3PO2, (ii) KMnO4 - KOH, $$\Delta$$ or (i) CH3 - CH2 - OH, (ii) KMnO4 - KOH, $$\Delta$$
Comments (0)


