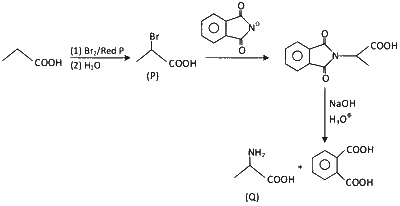
JEE Advance - Chemistry (2022 - Paper 1 Online - No. 13)
Considering the reaction sequence given below, the correct statement(s) is(are)

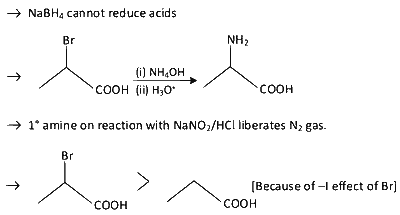
$\mathbf{P}$ can be reduced to a primary alcohol using $\mathrm{NaBH}_{4}$.
Treating $\mathbf{P}$ with conc. $\mathrm{NH}_{4} \mathrm{OH}$ solution followed by acidification gives $\mathbf{Q}$.
Treating $\mathbf{Q}$ with a solution of $\mathrm{NaNO}_{2}$ in aq. $\mathrm{HCl}$ liberates $\mathrm{N}_{2}$.
$\mathbf{P}$ is more acidic than $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COOH}$.
Explanation
Comments (0)


