JEE Advance - Chemistry (2022 - Paper 1 Online - No. 7)
The weight percentage of hydrogen in $\mathbf{Q}$, formed in the following reaction sequence, is ________.

[Given: Atomic mass of $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{~S}=32, \mathrm{Cl}=35$ ]
Answer
1.31
Explanation
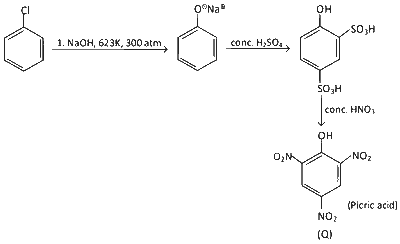
Formula of compound = C6H3N3O7
Molar Mass of compound = (12 $$\times$$ 6 + 3 + 14 $$\times$$ 3 + 16 $$\times$$ 7) g = 229 g
Weight % of H = $$\frac{3}{229}\times100=1.31$$
Comments (0)


