JEE Advance - Chemistry (2022 - Paper 1 Online - No. 3)
A solution is prepared by mixing $0.01 \mathrm{~mol}$ each of $\mathrm{H}_{2} \mathrm{CO}_{3}, \mathrm{NaHCO}_{3}, \mathrm{Na}_{2} \mathrm{CO}_{3}$, and $\mathrm{NaOH}$ in $100 \mathrm{~mL}$ of water. $p \mathrm{H}$ of the resulting solution is _________.
[Given: $p \mathrm{~K}_{\mathrm{a} 1}$ and $p \mathrm{~K}_{\mathrm{a} 2}$ of $\mathrm{H}_{2} \mathrm{CO}_{3}$ are $6.37$ and 10.32, respectively; $\log 2=0.30$ ]
Explanation
First acid base reaction between H2CO3 and NaOH takes place.
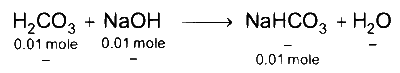
In the final solution, we have 0.01 mole Na2CO3 and 0.02 moles of NaHCO3.
Here, we have a buffer of NaHCO3 and Na2CO3.
$$\therefore$$ $$pH = p{K_{{a_2}}} + \log {{[Salt]} \over {[Acid]}}$$
$$ = 10.32 + \log {{\left( {{{0.01} \over {0.1}}} \right)} \over {\left( {{{0.02} \over {0.1}}} \right)}}$$
$$ = 10.32 + \log {1 \over 2}$$
$$ = 10.32 - \log 2$$
$$ = 10.32 - 0.3$$
$$ = 10.02$$
$$\therefore$$ $$pH = 10.02$$
Comments (0)


