JEE Advance - Chemistry (2022 - Paper 1 Online - No. 6)
Consider the following reaction.

On estimation of bromine in $1.00 \mathrm{~g}$ of $\mathbf{R}$ using Carius method, the amount of $\mathrm{AgBr}$ formed (in $\mathrm{g}$ ) is ___________.
[Given: Atomic mass of $\mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{P}=31, \mathrm{Br}=80, \mathrm{Ag}=108]$
Answer
1.50
Explanation
$$2P+3Br_2\to2PBr_3$$
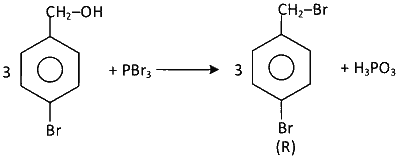
Number of moles in 1 gm of (R) = $$\frac{1}{250}$$
Number of moles of AgBr formed from (R) = $$\frac{2}{250}$$
Mass of AgBr formed $$=\frac{2\times188}{250}=1.50$$ gm
Comments (0)


