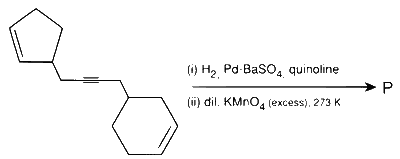
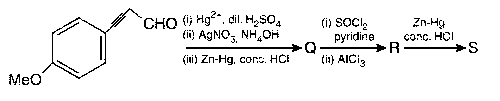JEE Advance - Chemistry (2019 - Paper 2 Offline)
4
Consider the following reactions (unbalanced).
$$Zn + Hot\,conc.\,{H_2}S{O_4}\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} G + R + X$$
$$Zn + conc.\,NaOH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} T + Q$$
$$G + {H_2}S + N{H_4}OH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} Z\,(a\,precipitate) + X + Y$$
Choose the correct option(s).
$$Zn + Hot\,conc.\,{H_2}S{O_4}\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} G + R + X$$
$$Zn + conc.\,NaOH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} T + Q$$
$$G + {H_2}S + N{H_4}OH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} Z\,(a\,precipitate) + X + Y$$
Choose the correct option(s).
Answer
B
C
D
9
The decomposition reaction
$$2{N_2}{O_5}(g)\buildrel \Delta \over \longrightarrow 2{N_2}{O_4}(g) + {O_2}(g)$$
is started in a closed cylinder under isothermal isochoric condition at an initial pressure of 1 atm. After Y $$ \times $$ 103 s, the pressure inside the cylinder is found to be 1.45 atm. If the rate constant of the reaction is 5 $$ \times $$ 10-4s-1, assuming ideal gas behaviour, the value of Y is ...............
$$2{N_2}{O_5}(g)\buildrel \Delta \over \longrightarrow 2{N_2}{O_4}(g) + {O_2}(g)$$
is started in a closed cylinder under isothermal isochoric condition at an initial pressure of 1 atm. After Y $$ \times $$ 103 s, the pressure inside the cylinder is found to be 1.45 atm. If the rate constant of the reaction is 5 $$ \times $$ 10-4s-1, assuming ideal gas behaviour, the value of Y is ...............
Answer
2.3
15
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the nth orbit of the atom and List-II contains options showing how they depend on n.
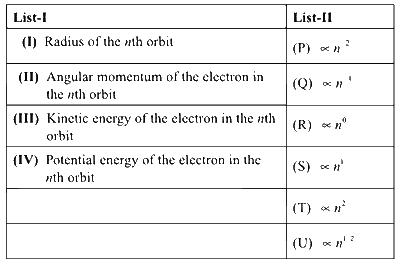
Which of the following options has the correct combination considering List-I and List-II?

Which of the following options has the correct combination considering List-I and List-II?
Answer
(A)
(III), (P)
16
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the nth orbit of the atom and List-II contains options showing how they depend on n.
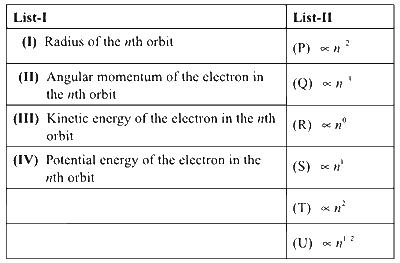
Which of the following options has the correct combination considering List-I and List-II?

Which of the following options has the correct combination considering List-I and List-II?
Answer
(C)
(I), (T)