JEE Advance - Chemistry (2019 - Paper 2 Offline - No. 7)
The cyanide process of gold extraction involves leaching out gold from its ore with CN$$-$$ in the presence of Q in water to form R. Subsequently, R is treated with T to obtain Au and Z. Choose the correct option(s).
Q is O2
Z is [Zn(CN)4]2$$-$$
T is Zn
R is [Au(CN)4]$$-$$
Explanation
Cyanide process of gold extraction involves leaching out gold from its ore with CN- in the presence of O2 (Q) in water to form [Au(CN)2]- (R). When [Au(CN)2]- reacts with Zn (T), it forms [Zn(CN)4]2- (Z) and Au.
The corresponding reactions are as follows :
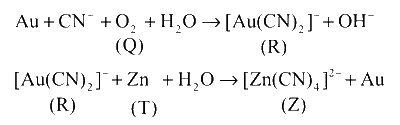
Hence, options (a, b, c) are correct.
The corresponding reactions are as follows :
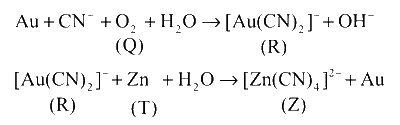
Hence, options (a, b, c) are correct.
Comments (0)


