JEE Advance - Chemistry (2019 - Paper 2 Offline - No. 12)
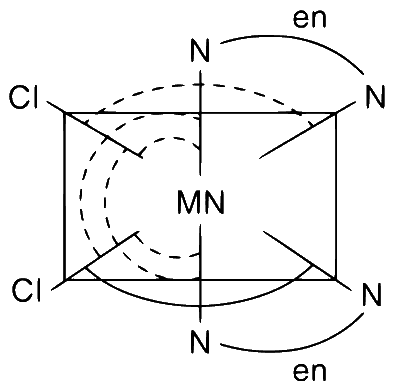
Total number of $$cis\,N - Mn - Cl$$ bond angles (that is $$Mn - N$$ and $$Mn - Cl$$ bonds in cis positions) present in a molecule of $$cis[Mn{(en)_2}C{l_2}]$$ complex is ..................
(en = NH2CH2CH2NH2)
(en = NH2CH2CH2NH2)
Answer
6
Explanation
[Mn(en)2Cl2] structure is shown below having 6 cis
N-Mn-Cl bond angles. The dotted markings show the cis
N-Mn-Cl bond angles.

Comments (0)


