JEE Advance - Chemistry (2019 - Paper 2 Offline - No. 4)
Consider the following reactions (unbalanced).
$$Zn + Hot\,conc.\,{H_2}S{O_4}\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} G + R + X$$
$$Zn + conc.\,NaOH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} T + Q$$
$$G + {H_2}S + N{H_4}OH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} Z\,(a\,precipitate) + X + Y$$
Choose the correct option(s).
$$Zn + Hot\,conc.\,{H_2}S{O_4}\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} G + R + X$$
$$Zn + conc.\,NaOH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} T + Q$$
$$G + {H_2}S + N{H_4}OH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} Z\,(a\,precipitate) + X + Y$$
Choose the correct option(s).
The oxidation state of Zn in T is +1
R is a V-shaped molecule
Bond order of Q is 1 in its ground state
Z is dirty white in colour
Explanation
When Zn react with hot conc. H2SO4 then SO2 is released and ZnSO4 is obtained.
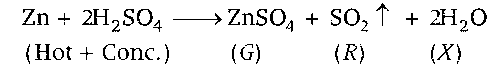
R(SO2) molecule is V-shaped
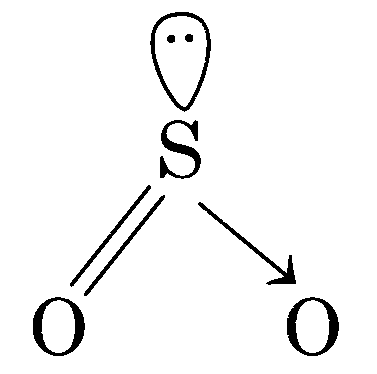
Thus, option (b) is correct.
When Zn is react with conc. NaOH then H2 gas is evolved and Na2ZnO2 is obtained.
$$Zn + 2NaOH(conc.)\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} \mathop {N{a_2}Zn{O_2}}\limits_{(T)} + \mathop {{H_2}}\limits_{(Q)} \uparrow $$
In ground state, H$$-$$H (Q)
(bond order = 1)
Thus, option (c) is correct.
The oxidation state of Zn in T(Na2ZnO2) is +2
Thus, option (a) is incorrect.
$$\mathop {ZnS{O_4}}\limits_{(G)} + {H_2}S + N{H_4}OH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} \mathop {ZnS}\limits_{(Z)} \downarrow + \mathop {2{H_2}O}\limits_{(X)} + \mathop {{{(N{H_4})}_2}S{O_4}}\limits_{(Y)} $$
ZnS (Z) compound is dirty white coloured.
Thus, option (d) is correct.
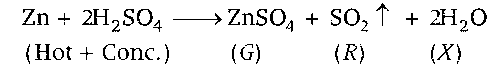
R(SO2) molecule is V-shaped

Thus, option (b) is correct.
When Zn is react with conc. NaOH then H2 gas is evolved and Na2ZnO2 is obtained.
$$Zn + 2NaOH(conc.)\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} \mathop {N{a_2}Zn{O_2}}\limits_{(T)} + \mathop {{H_2}}\limits_{(Q)} \uparrow $$
In ground state, H$$-$$H (Q)
(bond order = 1)
Thus, option (c) is correct.
The oxidation state of Zn in T(Na2ZnO2) is +2
Thus, option (a) is incorrect.
$$\mathop {ZnS{O_4}}\limits_{(G)} + {H_2}S + N{H_4}OH\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} \mathop {ZnS}\limits_{(Z)} \downarrow + \mathop {2{H_2}O}\limits_{(X)} + \mathop {{{(N{H_4})}_2}S{O_4}}\limits_{(Y)} $$
ZnS (Z) compound is dirty white coloured.
Thus, option (d) is correct.
Comments (0)


