JEE Advance - Chemistry (2019 - Paper 2 Offline - No. 16)
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the nth orbit of the atom and List-II contains options showing how they depend on n.
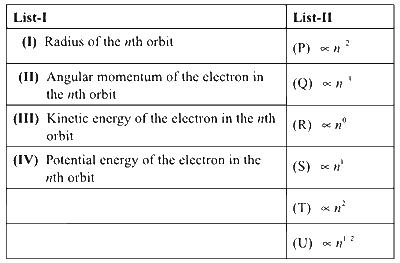
Which of the following options has the correct combination considering List-I and List-II?

Which of the following options has the correct combination considering List-I and List-II?
(II), (R)
(I), (P)
(I), (T)
(II), (Q)
Explanation
(I) Radius of the nth orbit, r = $$0.529 \times {{{n^2}} \over Z}$$
Here, $$r \propto {n^2}$$
From List-II, correct match is (I, T)
(II) Angular momentum of the electron, $$mvr = {{nh} \over {2\pi }}$$ or $$mvr \propto n$$
From List - II, correct match (II, S)
Hence, correct matching from List-I and List-II on the basis of given options is (I, T).
Here, $$r \propto {n^2}$$
From List-II, correct match is (I, T)
(II) Angular momentum of the electron, $$mvr = {{nh} \over {2\pi }}$$ or $$mvr \propto n$$
From List - II, correct match (II, S)
Hence, correct matching from List-I and List-II on the basis of given options is (I, T).
Comments (0)


