JEE Advance - Chemistry (2019 - Paper 2 Offline - No. 5)
With reference to aqua-regia, choose the correct option(s).
Aqua-regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v / v) ratio
The yellow colour of aqua-regia is due to the presence of NOCl and Cl2
Reaction of gold with aqua-regia produces an anion having Au in +3 oxidation state
Reaction of gold with aqua regia produces NO2 in the absence of air
Explanation
The explanation of given statements are as follows :
(a) Aqua-regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratio and is used in oxidation of gold and platinum. Hence, option (a) is correct.
(b) Yellow colour of aqua-regia is due to its decomposition into NOCl (orange yellow) and Cl2 (greenish yellow). Hence, option (b) is correct.
(c) When gold reacts with aqua-regia then it produces $$AuCl_4^ - $$ anion complex in which Au has +3 oxidation state.
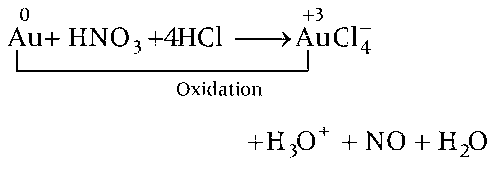
Hence, option (c) is correct.
(d) Reaction of gold with aqua-regia produces NO gas in absence of air. Hence, option (d) is incorrect.
(a) Aqua-regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratio and is used in oxidation of gold and platinum. Hence, option (a) is correct.
(b) Yellow colour of aqua-regia is due to its decomposition into NOCl (orange yellow) and Cl2 (greenish yellow). Hence, option (b) is correct.
(c) When gold reacts with aqua-regia then it produces $$AuCl_4^ - $$ anion complex in which Au has +3 oxidation state.

Hence, option (c) is correct.
(d) Reaction of gold with aqua-regia produces NO gas in absence of air. Hence, option (d) is incorrect.
Comments (0)


