JEE Advance - Chemistry (2019 - Paper 2 Offline - No. 2)
Choose the correct option(s) that give(s) an aromatic compound as the major product.
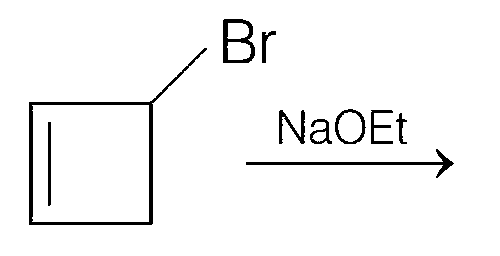
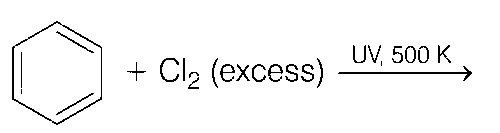
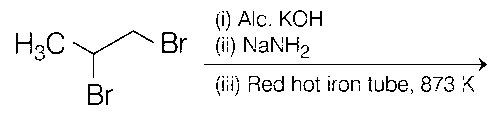
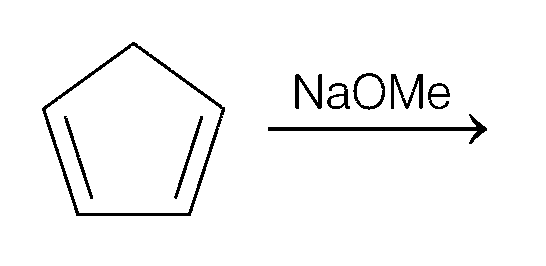
Explanation
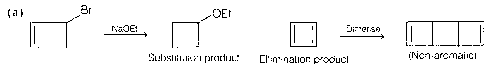
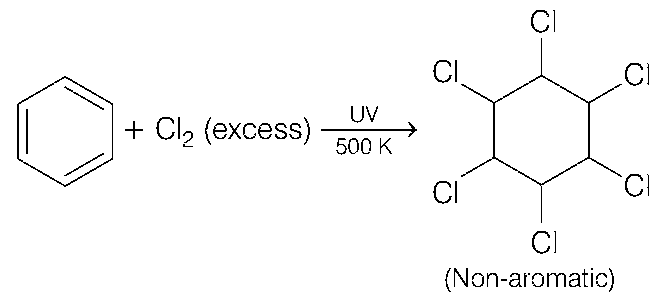
(b) Benzene react with Cl2 (excess) in presence of UV light and 500 K of temperature to form benzene hexachloride (non-aromatic).
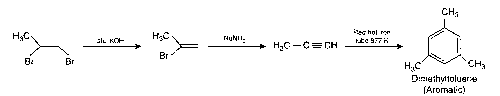
(c)
(d)
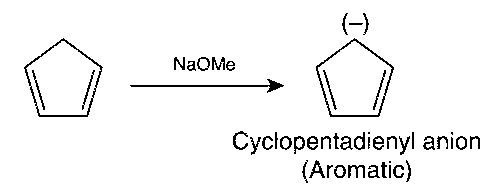
Thus, (c) and (d) options are correct.
Comments (0)


