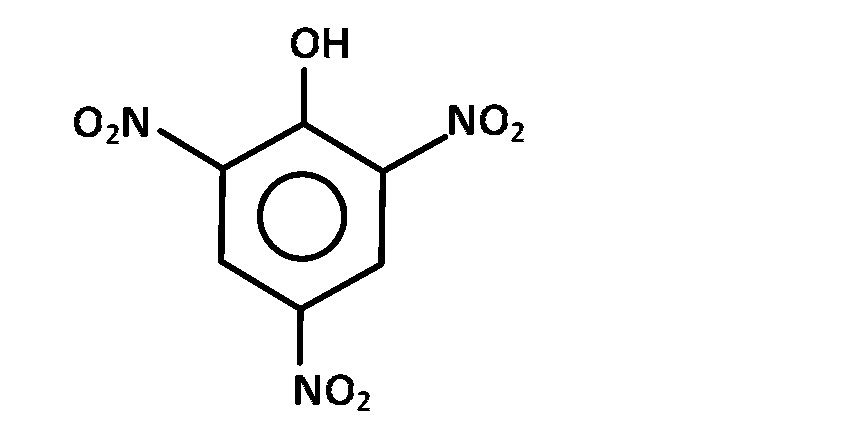
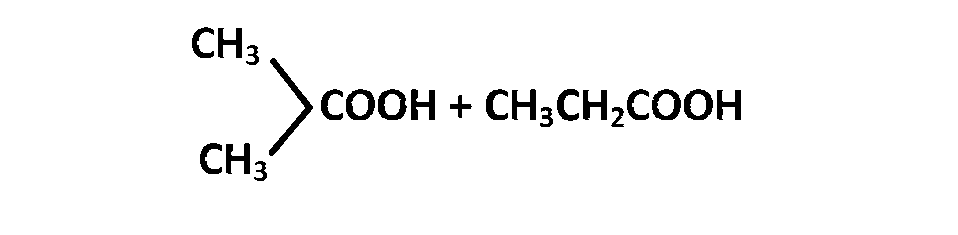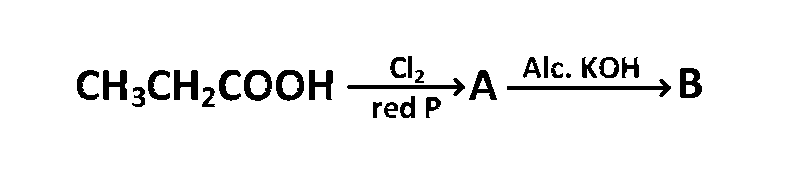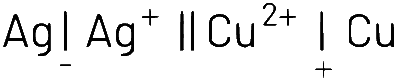JEE MAIN - Chemistry (2002)
32
For the following cell with hydrogen electrodes at two different pressure p1 and p2. What will be the emf for the given cell :
$$\eqalign{ & Pt({H_2})|{H^ + }(aq)|Pt({H_2}) \cr & \,\,\,\,\,{p_1}\,\,\,\,\,\,\,\,\,\,\,\,\,\,1M\,\,\,\,\,\,\,\,\,\,\,\,{p_2} \cr} $$
$$\eqalign{ & Pt({H_2})|{H^ + }(aq)|Pt({H_2}) \cr & \,\,\,\,\,{p_1}\,\,\,\,\,\,\,\,\,\,\,\,\,\,1M\,\,\,\,\,\,\,\,\,\,\,\,{p_2} \cr} $$
Answer
(B)
$${{RT} \over 2F}{\log _e}{{{P_1}} \over {{P_2}}}$$