JEE MAIN - Chemistry (2002 - No. 45)
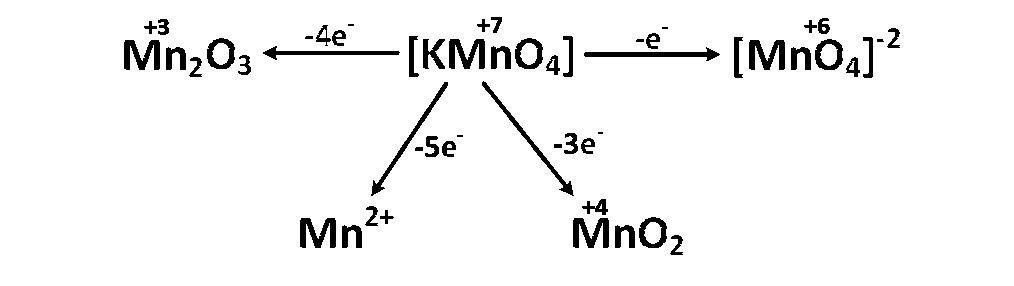
When KMnO4 acts as an oxidising agent and ultimately forms [MnO4]-2, MnO2, Mn2O3, Mn+2
then the number of electrons transferred in each case respectively is :
4, 3, 1, 5
1, 5, 3, 7
1, 3, 4, 5
3, 5, 7, 1
Explanation
Comments (0)


