JEE MAIN - Chemistry (2002 - No. 57)
In which of the following species the interatomic bond angle is 109o28' ?
NH3, BF4-
NH4+, BF4-
NH3, BF3
NH2-1, BF3
Explanation
Bond angle 109o 28' means Regular Tetrahedral geometry and hybridization is sp3.
Steric Number (SN) for sp3 hybridization is 4.
In sp3 molecules can have different shapes which is decided by SN number
(1) $$\,\,\,\,$$ 4 bond pair in a molecule then angle between bonds 109o 28'
(2) $$\,\,\,\,$$ 3 bond pair and 1 lone pair in a molecule then angle between bonds 107o.
(3) $$\,\,\,\,$$ 2 bond pair and 2 lone pair in a molecule then angle between bonds 104.5o
(4) $$\,\,\,\,$$ 1 bond pair and 3 lone pair in a molecule then bond angle is undefined as there is only one bond.
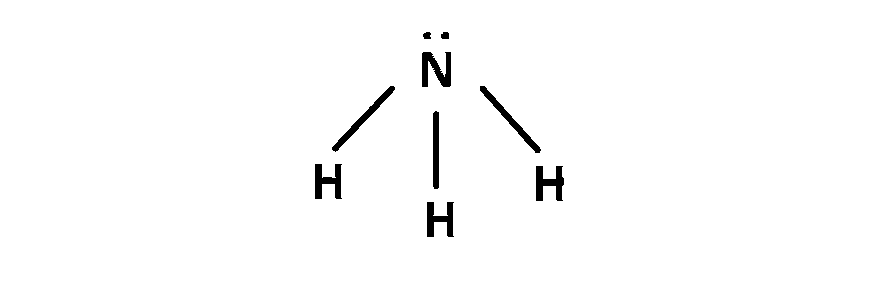
(1) $$\,\,\,\,$$ In NH3, 3 Bond pair(BP) +1 lone pair (LP) present so angle between bond 107o.
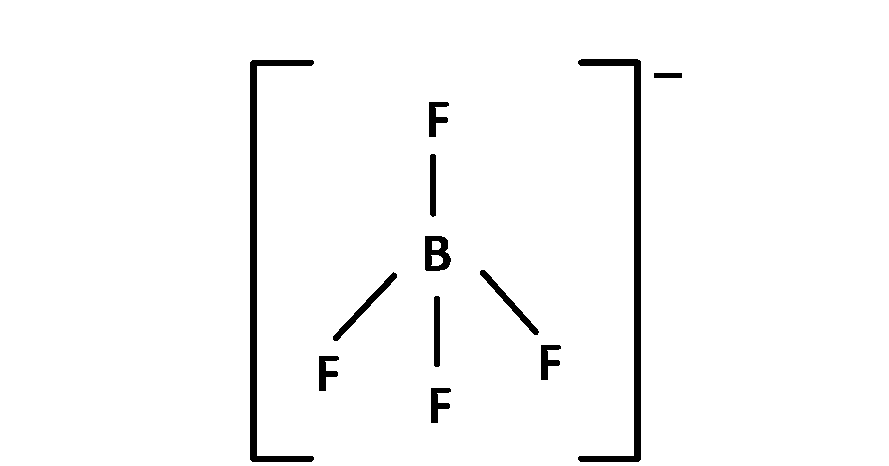
(2) $$\,\,\,\,$$ BF$$_4^ - $$, 4 bond pair present so angle is 109o 28'.
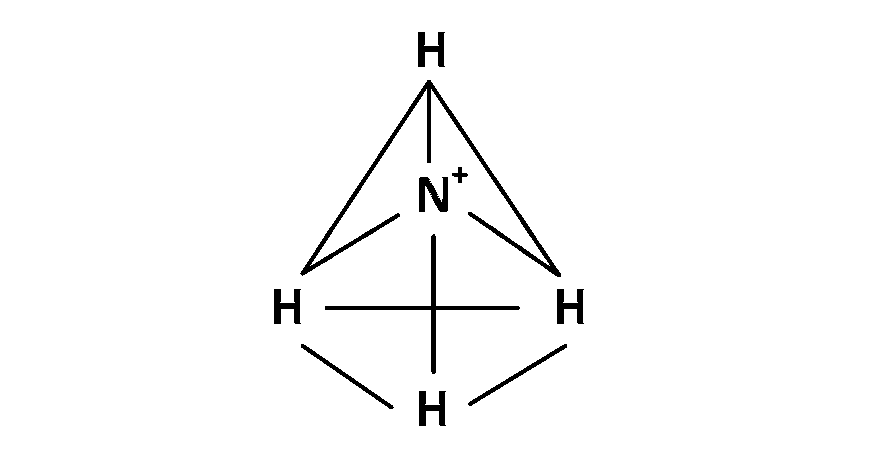
(3) In NH$$_4^ + $$, 4 bond pair present so angle between bond is 109o 28'
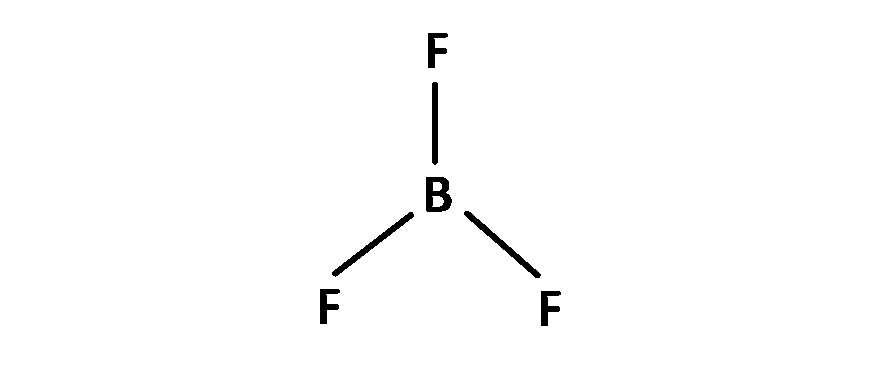
(4) $$\,\,\,\,$$ BF3 has sp2 hybridization. So bond angle is 120o.
(5) $$\,\,\,\,$$ In NH$$_2^ - $$, 2 bond pair and 2 lone pair present, so bond angle is 104.5o
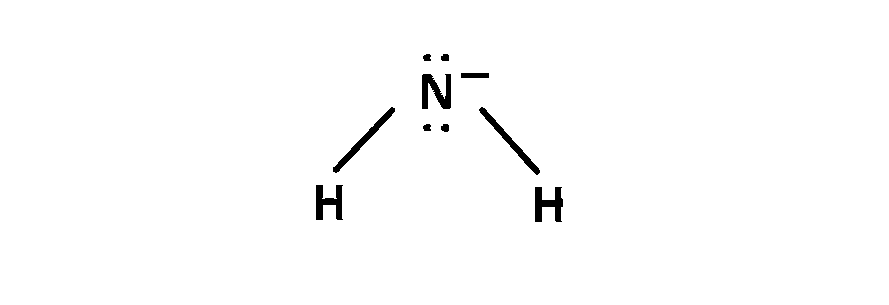
So, $$NH{}^ + $$ and BF$$_4^ - $$ has bond angle 109o 28'.
Steric Number (SN) for sp3 hybridization is 4.
In sp3 molecules can have different shapes which is decided by SN number
(1) $$\,\,\,\,$$ 4 bond pair in a molecule then angle between bonds 109o 28'
(2) $$\,\,\,\,$$ 3 bond pair and 1 lone pair in a molecule then angle between bonds 107o.
(3) $$\,\,\,\,$$ 2 bond pair and 2 lone pair in a molecule then angle between bonds 104.5o
(4) $$\,\,\,\,$$ 1 bond pair and 3 lone pair in a molecule then bond angle is undefined as there is only one bond.
(1) $$\,\,\,\,$$ In NH3, 3 Bond pair(BP) +1 lone pair (LP) present so angle between bond 107o.

(2) $$\,\,\,\,$$ BF$$_4^ - $$, 4 bond pair present so angle is 109o 28'.

(3) In NH$$_4^ + $$, 4 bond pair present so angle between bond is 109o 28'

(4) $$\,\,\,\,$$ BF3 has sp2 hybridization. So bond angle is 120o.

(5) $$\,\,\,\,$$ In NH$$_2^ - $$, 2 bond pair and 2 lone pair present, so bond angle is 104.5o

So, $$NH{}^ + $$ and BF$$_4^ - $$ has bond angle 109o 28'.
Comments (0)


