JEE MAIN - Chemistry (2002 - No. 14)
In which of the following species is the underlined carbon having sp3 hybridisation?
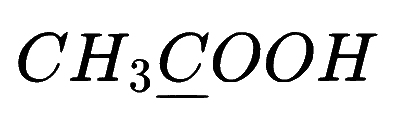
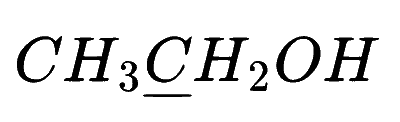
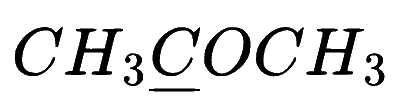
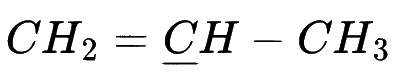
Explanation
(a) $$\,\,\,\,$$
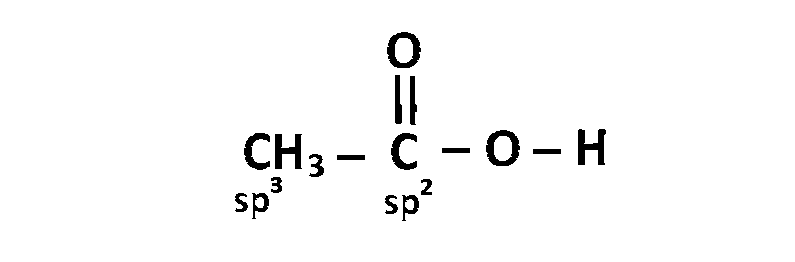
(b) $$\,\,\,\,$$
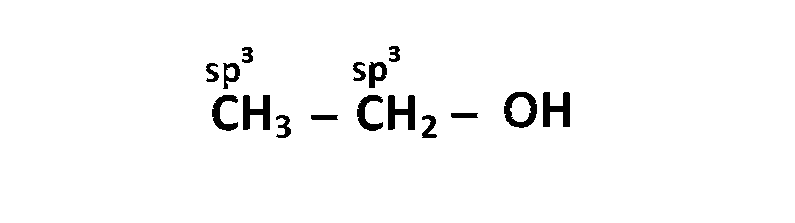
(c) $$\,\,\,\,$$
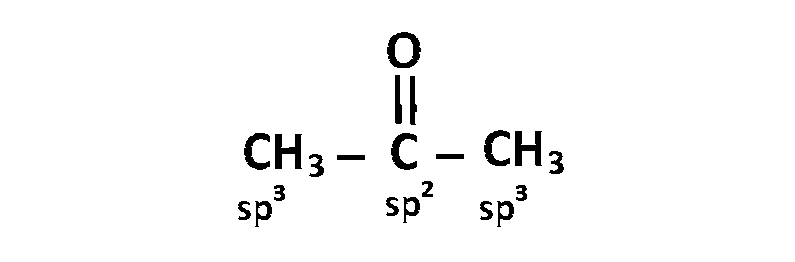
(d) $$\,\,\,\,$$
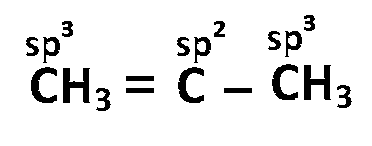
So, option (B) is correct
Note :
If in carbon(C) all 4 bonds are sigma bond then it is sp3 hybridization and if there is 3 sigma bond then sp2 and if there is only 2 sigma bond there sp hybridization.
Comments (0)


