JEE MAIN - Chemistry (2002 - No. 26)
The correct order of ionic radius is
$$Ce > Sm > Tb > Lu$$
$$Lu > Tb > Sm > Ce$$
$$Tb > Lu > Sm > Ce$$
$$Sm > Tb > Lu > Ce$$
Explanation
Those are the elements of lanthanides series.
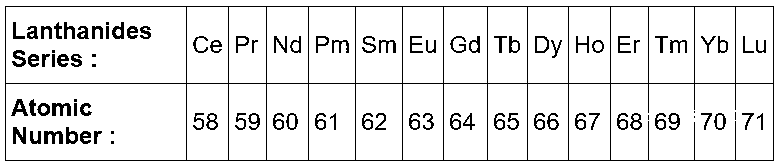
In lanthanide series the extra electron is added in $$4f$$ subshell and we know in periodic table from left to right the effective nuclear charge increases as electron is added so the size of elements will decreases.
So, the correct order is $$Ce > Sm > Tb > Lu.$$

In lanthanide series the extra electron is added in $$4f$$ subshell and we know in periodic table from left to right the effective nuclear charge increases as electron is added so the size of elements will decreases.
So, the correct order is $$Ce > Sm > Tb > Lu.$$
Comments (0)


