JEE MAIN - Chemistry (2002 - No. 48)
A square planar complex is formed by hybridisation of which atomic orbitals ?
s, px, py, dxy
s, px, py, dx2 - y2
s, px, py, dz2
s, px, py, dyz
Explanation
Hybridization of square planar complex is dsp2 .
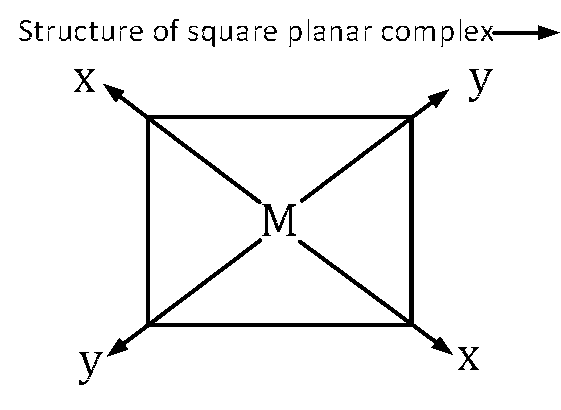
In square planar complex all 4 surrounding atoms and central atom are in the same plane and let this plane is x $$-$$ y plane. As it is dsp2 hybridized, so it has 1 d orbital, 1 s orbital and 2 p orbital.
s orbital is non-directional, so we just write it as s.
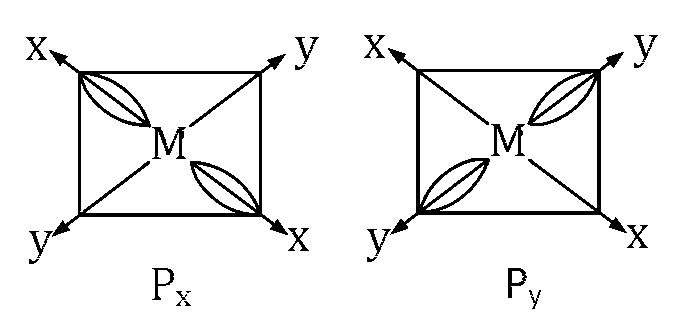
In px the two lobes are along x-axis and in py two lobes are along y-axis
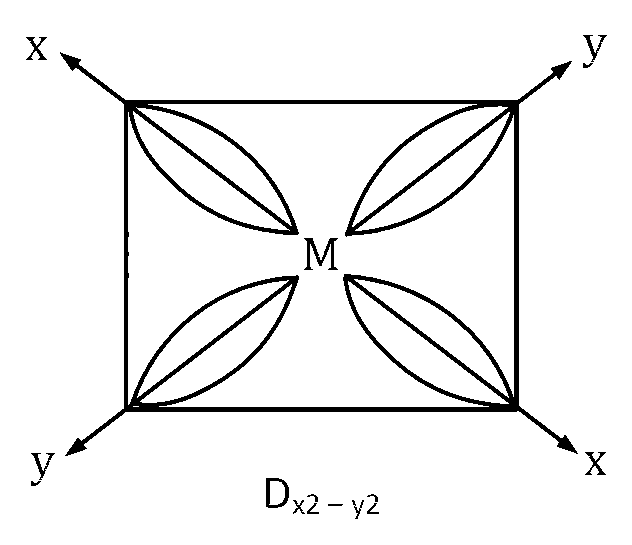
One d orbital is dx2 $$-$$ y2, it means out of four lobes of d orbital two along x-axis and two along y-axis.

In square planar complex all 4 surrounding atoms and central atom are in the same plane and let this plane is x $$-$$ y plane. As it is dsp2 hybridized, so it has 1 d orbital, 1 s orbital and 2 p orbital.
s orbital is non-directional, so we just write it as s.

In px the two lobes are along x-axis and in py two lobes are along y-axis
One d orbital is dx2 $$-$$ y2, it means out of four lobes of d orbital two along x-axis and two along y-axis.

Comments (0)


