JEE MAIN - Chemistry (2002 - No. 27)
$$C{e^{ + 3}},\,\,L{a^{ + 3}},\,\,P{m^{ + 3}}\,\,$$ and $$Y{b^{ + 3}}\,\,$$ have ionic radial in the increasing order as
$$L{a^{ + 3}}\,\, < C{e^{ + 3}}\,\, < P{m^{ + 3}}\,\, < Y{b^{ + 3}}$$
$$Y{b^{ + 3}}\,\, < P{m^{ + 3}}\,\, < C{e^{ + 3}}\,<\,L{a^{ + 3}}$$
$$L{a^{ + 3}}\,\, = C{e^{ + 3}}\,\, < P{m^{ + 3}}\,\, < Y{b^{ + 3}}$$
$$Y{b^{ + 3}}\,\, < P{m^{ + 3}}\,\, < L{a^{ + 3}}\,\, < C{e^{ + 3}}$$
Explanation
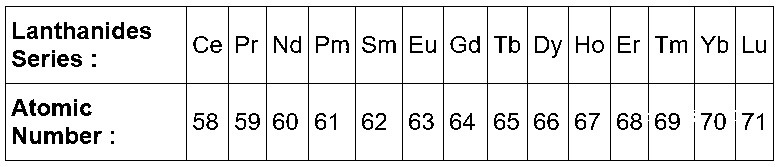
Here $$C{e^{ + 3}},\,\,P{m^{ + 3}},\,\,Y{b^{ + 3}}$$ are from lanthanide series.
$$L{a^{ + 3}}$$ atomic number is $$57.$$ This is a $$d$$ block element but all the property of $$La$$ is similar to lanthanides. It is present before $$Ce.$$
As we know in lanthanides the radius of elements decreases from left to right of periodic table.
So, order will be $$L{a^{ + 3}} > C{e^{ + 3}} > P{m^{ + 3}} > Y{b^{ + 3}}$$
Comments (0)


