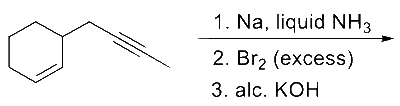
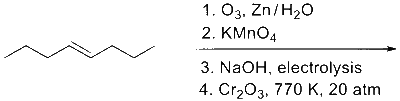
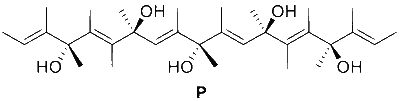
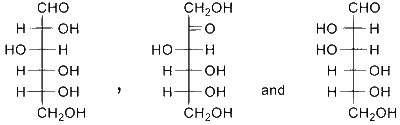
JEE Advance - Chemistry (2022 - Paper 2 Online)
1
Concentration of $\mathrm{H}_{2} \mathrm{SO}_{4}$ and $\mathrm{Na}_{2} \mathrm{SO}_{4}$ in a solution is $1 \mathrm{M}$ and $1.8 \times 10^{-2} \mathrm{M}$, respectively. Molar solubility of $\mathrm{PbSO}_{4}$ in the same solution is $\mathrm{X} \times 10^{-\mathrm{Y}} \mathrm{M}$ (expressed in scientific notation). The value of $Y$ is ________.
[Given: Solubility product of $\mathrm{PbSO}_{4}\left(K_{s p}\right)=1.6 \times 10^{-8}$. For $\mathrm{H}_{2} \mathrm{SO}_{4}, K_{a l}$ is very large and $\left.K_{a 2}=1.2 \times 10^{-2}\right]$
Answer
6
2
An aqueous solution is prepared by dissolving $0.1 \mathrm{~mol}$ of an ionic salt in $1.8 \mathrm{~kg}$ of water at $35^{\circ} \mathrm{C}$. The salt remains $90 \%$ dissociated in the solution. The vapour pressure of the solution is $59.724 \mathrm{~mm}$ of Hg. Vapor pressure of water at $35{ }^{\circ} \mathrm{C}$ is $60.000 \mathrm{~mm}$ of $\mathrm{Hg}$. The number of ions present per formula unit of the ionic salt is _________.
Answer
5
3
Consider the strong electrolytes $Z_{m} X_{n}, U_{m} Y_{p}$ and $V_{m} X_{n}$. Limiting molar conductivity ( $\Lambda^{0}$ ) of $\mathrm{U}_{\mathrm{m}} \mathrm{Y}_{\mathrm{p}}$ and $\mathrm{V}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}}$ are 250 and $440 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$, respectively. The value of $(\mathrm{m}+\mathrm{n}+\mathrm{p})$ is
Given:
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.
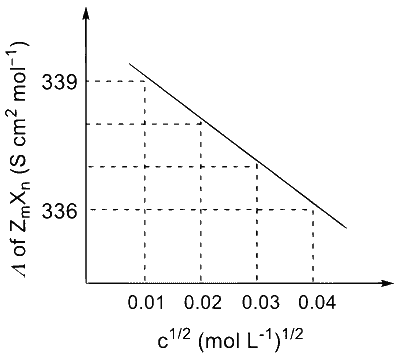
Given:
| Ion | $\mathrm{Z}^{\mathrm{n}+}$ | $\mathrm{U}^{\mathrm{p}+}$ | $\mathrm{V}^{\mathrm{n}+}$ | $\mathrm{X}^{\mathrm{m}-}$ | $\mathrm{Y}^{\mathrm{m}-}$ |
|---|---|---|---|---|---|
| $\lambda^{0}\left(\mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}\right)$ | $50.0$ | $25.0$ | $100.0$ | $80.0$ | $100.0$ |
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.

Answer
7
9
To check the principle of multiple proportions, a series of pure binary compounds $\left(\mathrm{P}_{\mathrm{m}} \mathrm{Q}_{\mathrm{n}}\right)$ were analyzed and their composition is tabulated below. The correct option(s) is(are)
| Compound | Weight % of $\mathrm{P}$ | Weight % of $\mathrm{Q}$ |
|---|---|---|
| 1 | 50 | 50 |
| 2 | 44.4 | 55.6 |
| 3 | 40 | 60 |
Answer
B
C
17
The reaction of $\mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}$ and $\mathrm{NaCl}$ in water produces a precipitate that dissolves upon the addition of $\mathrm{HCl}$ of appropriate concentration. The dissolution of the precipitate is due to the formation of
Answer
(C)
$\left[\mathrm{PbCl}_{4}\right]^{2-}$