JEE Advance - Chemistry (2022 - Paper 2 Online - No. 13)
Considering the following reaction sequence, the correct statement(s) is(are)


Compounds $\mathbf{P}$ and $\mathbf{Q}$ are carboxylic acids.
Compound $\mathbf{S}$ decolorizes bromine water.
Compounds $\mathbf{P}$ and $\mathbf{S}$ react with hydroxylamine to give the corresponding oximes.
Compound $\mathbf{R}$ reacts with dialkylcadmium to give the corresponding tertiary alcohol.
Explanation
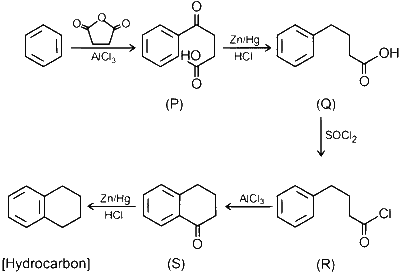
Compound (S) can not decolorizes bromine water.
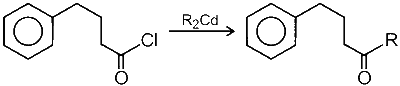
R2Cd is a less reactive nucleophile so reaction stops at carbonyl group.
Comments (0)


