JEE Advance - Chemistry (2022 - Paper 2 Online - No. 5)
Thermal decomposition of $\mathrm{AgNO}_{3}$ produces two paramagnetic gases. The total number of electrons present in the antibonding molecular orbitals of the gas that has the higher number of unpaired electrons is ____________.
Answer
6
Explanation
$$ 2 \mathrm{AgNO}_3(\mathrm{~s}) \stackrel{\Delta}{\longrightarrow} 2 \mathrm{Ag}(\mathrm{s})+2 \mathrm{NO}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) $$
Both the $\mathrm{NO}_2$ and $\mathrm{O}_2$ gases are paramagnetic. $\mathrm{NO}_2(\mathrm{~g})$ has 1 unpaired electron and $\mathrm{O}_2(\mathrm{~g})$ has 2 unpaired electrons.
According to MOT,
$$\therefore\,\,\,\,$$ Molecular orbital configuration of O2 (16 electrons) is$${\sigma _{1{s^2}}}\,\,\sigma _{1{s^2}}^ * \,$$ $${\sigma _{2{s^2}}}\,\,\sigma _{2{s^2}}^ * \,$$ $${\sigma _{2p_z^2}}\,\,{\pi _{2p_x^2}} = {\pi _{2p_y^2}}\,\,\pi _{2p_x^1}^ * \,\, = \pi _{2p_y^1}^ * $$
$$\therefore\,\,\,\,$$Na = Anti bonding electrons = 6
Nb = 10
Note :
Nb = Number of electrons in bonding molecular orbital
Na $$=$$ Number of electrons in anti bonding molecular orbital
(1) $$\,\,\,\,$$ upto 14 electrons, molecular orbital configuration is
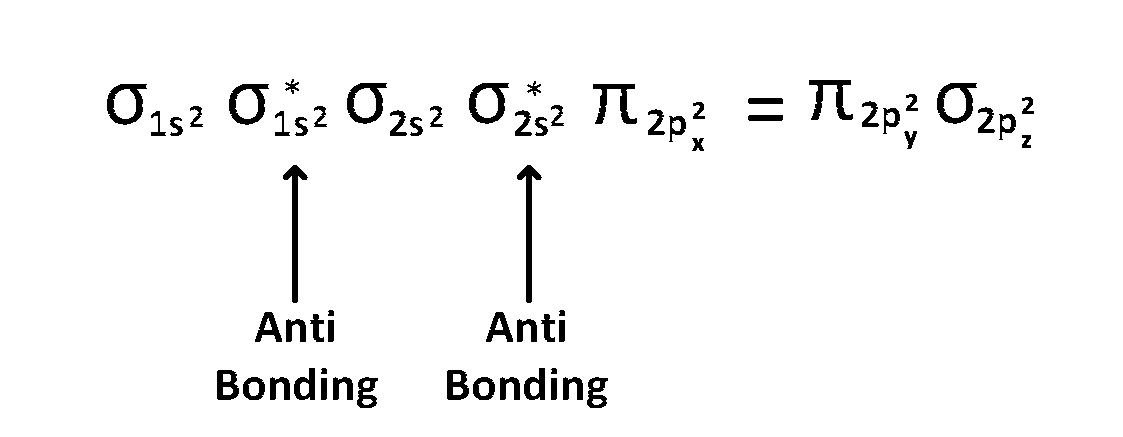
Here Na = Anti bonding electrons $$=$$ 4 and Nb = 10
(2) $$\,\,\,\,$$ After 14 electrons to 20 electrons molecular orbital configuration is - - -

Here Na = 10
and Nb = 10
Comments (0)


