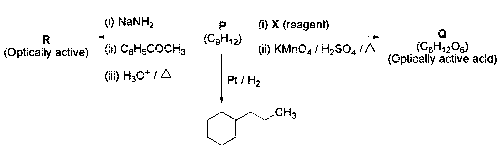
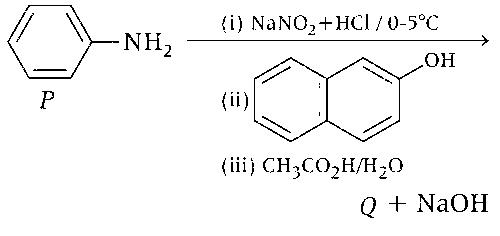
JEE Advance - Chemistry (2020 - Paper 2 Offline)
4
An acidified solution of potassium chromate was layered with an equal volume of amyl alcohol. When it was shaken after the addition of 1 mL of 3% H2O2, a blue alcohol layer was obtained. The blue colour is due to the formation of a chromium (VI) compound 'X'. What is the number of oxygen atoms bonded to chromium through only single bond in a molecule of X?
Answer
4
5
The structure of a peptide is given below.
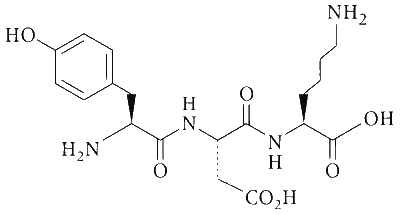
If the absolute values of the net charge of the peptide at $\mathrm{pH}$ $=2$, $\mathrm{pH}=6$, and $\mathrm{pH}=11$ are $\left|Z_1\right|,\left|Z_2\right|$, and $\left|Z_3\right|$, respectively, then what is $\left|Z_1\right|+\left|Z_2\right|+\left|Z_3\right|$?

If the absolute values of the net charge of the peptide at $\mathrm{pH}$ $=2$, $\mathrm{pH}=6$, and $\mathrm{pH}=11$ are $\left|Z_1\right|,\left|Z_2\right|$, and $\left|Z_3\right|$, respectively, then what is $\left|Z_1\right|+\left|Z_2\right|+\left|Z_3\right|$?
Answer
5
7
In an experiment, m grams of a compound X (gas/liquid/solid) taken in a container is loaded in a balance as shown in figure I below.
In the presence of a magnetic field, the pan with X is either deflected upwards (figure II), or deflected downwards (figure III), depending on the compound X. Identify the correct statement(s).
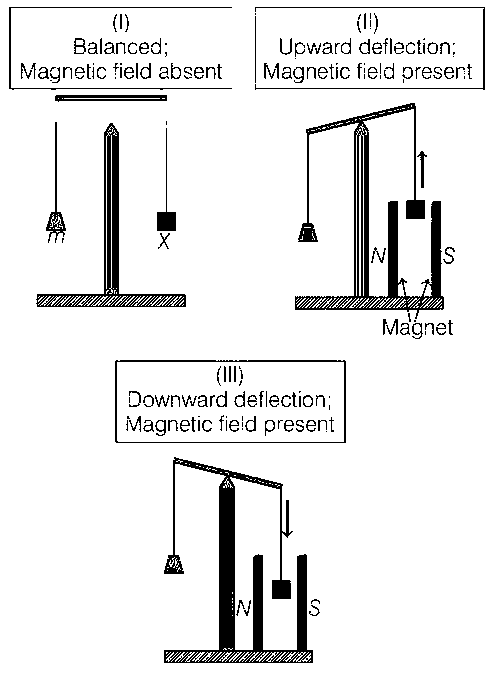
In the presence of a magnetic field, the pan with X is either deflected upwards (figure II), or deflected downwards (figure III), depending on the compound X. Identify the correct statement(s).

Answer
A
B
C
13
A solution of 0.1 M weak base (B) is titrated with 0.1 M of a strong acid (HA). The variation of pH of the solution with the volume of HA added is shown in the figure below. What is the pKb of the base? The neutralisation reaction is given by
$$B + HA\buildrel {} \over \longrightarrow B{H^ + } + {A^ - }$$
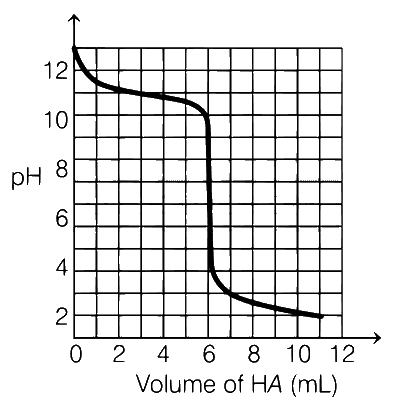
$$B + HA\buildrel {} \over \longrightarrow B{H^ + } + {A^ - }$$

Answer
3
15
The figure below is the plot of potential energy versus internuclear distance (d) of H2 molecule in the electronic ground state. What is the value of the net potential energy E0 (as indicated in the figure) in kJ mol-1, for d = d0 at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent? As reference, the potential energy of H atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as 6.023 $$ \times $$ 1023 mol-1.
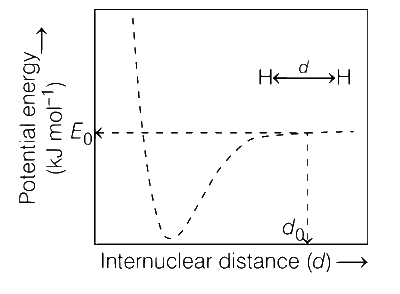
Use Avogadro constant as 6.023 $$ \times $$ 1023 mol-1.

Answer
-5242.41
Or +1 more17
Tin is obtained from cassiterite by reduction with coke. Use the data given below to determine the minimum temperature (in K) at which the reduction of cassiterite by coke would take place.
At $$298K:{\Delta _f}H^\circ [Sn{O_2}(s)] = - 581.0$$ mol-1,
$$\eqalign{ & {\Delta _f}H^\circ [(C{O_2})(g)] = - 394.0\,kJ\,mol{ ^{-1}} \cr & S^\circ [Sn{O_2}(s)] = 56.0J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [Sn(s)] = 52.0\,J\,K{ ^{-1}}mo{l^{ - 1}} \cr & S^\circ [C(s)] = 6.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [C{O_2}(g)] = 210.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr} $$
Assume that, the enthalpies and the entropies are temperature independent.
At $$298K:{\Delta _f}H^\circ [Sn{O_2}(s)] = - 581.0$$ mol-1,
$$\eqalign{ & {\Delta _f}H^\circ [(C{O_2})(g)] = - 394.0\,kJ\,mol{ ^{-1}} \cr & S^\circ [Sn{O_2}(s)] = 56.0J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [Sn(s)] = 52.0\,J\,K{ ^{-1}}mo{l^{ - 1}} \cr & S^\circ [C(s)] = 6.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [C{O_2}(g)] = 210.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr} $$
Assume that, the enthalpies and the entropies are temperature independent.
Answer
935
Or +2 more18
An acidified solution of 0.05 M Zn2+ is saturated with 0.1 M H2S. What is the minimum molar concentration (M) of H+ required to prevent the precipitation of ZnS?
Use Ksp(ZnS) = 1.25 $$ \times $$ 10$$-$$22 and overall dissociation constant of
H2S, Knet = K1K2 = 1 $$ \times $$ 10-21.
Use Ksp(ZnS) = 1.25 $$ \times $$ 10$$-$$22 and overall dissociation constant of
H2S, Knet = K1K2 = 1 $$ \times $$ 10-21.
Answer
0.2
Or +1 more