JEE Advance - Chemistry (2020 - Paper 2 Offline - No. 15)
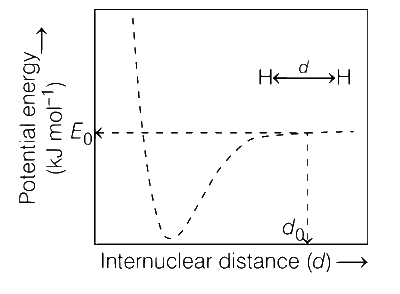
The figure below is the plot of potential energy versus internuclear distance (d) of H2 molecule in the electronic ground state. What is the value of the net potential energy E0 (as indicated in the figure) in kJ mol-1, for d = d0 at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent? As reference, the potential energy of H atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as 6.023 $$ \times $$ 1023 mol-1.
Use Avogadro constant as 6.023 $$ \times $$ 1023 mol-1.

Answer
-5242.41
-5242.4192
- OR
Explanation
Given that, electrons and nucleus are at infinite distance, so potential energy of H-atom is taken as zero.
Therefore, according to Bohr's model, potential energy of a H-atom with electron in its ground state = $$-$$27.2 eV
At d = d0, nucleus-nucleus and electron-electron repulsion is absent.
Hence, potential energy will be calculated for 2 H atoms = $$-$$2 $$ \times $$ 27.2 eV = $$-$$54.4 eV
Potential energy of 1 mol H atoms in kJ
= $${{54.4 \times 6.02 \times {{10}^{23}} \times 1.6 \times {{10}^{ - 19}}} \over {1000}}$$
= $$ - 5242.4192$$ kJ/mol
Therefore, according to Bohr's model, potential energy of a H-atom with electron in its ground state = $$-$$27.2 eV
At d = d0, nucleus-nucleus and electron-electron repulsion is absent.
Hence, potential energy will be calculated for 2 H atoms = $$-$$2 $$ \times $$ 27.2 eV = $$-$$54.4 eV
Potential energy of 1 mol H atoms in kJ
= $${{54.4 \times 6.02 \times {{10}^{23}} \times 1.6 \times {{10}^{ - 19}}} \over {1000}}$$
= $$ - 5242.4192$$ kJ/mol
Comments (0)


