JEE Advance - Chemistry (2020 - Paper 2 Offline - No. 8)
Which of the following plots is(are) correct for the given reaction?
([P]0 is the initial concentration of P)
([P]0 is the initial concentration of P)


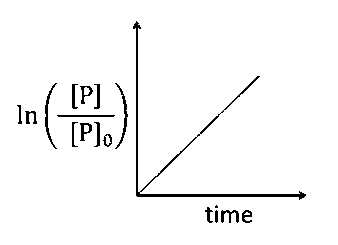
Explanation
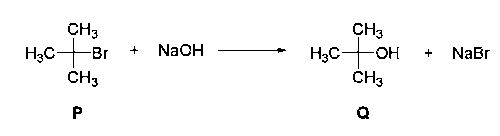
$${(C{H_3})_3}C - Br + NaOH\mathrel{\mathop{\kern0pt\longrightarrow}
\limits_{(First\,order\,reaction)}^{{S_{{N^1}}}}} {(C{H_3})_3}C - OH + NaBr$$
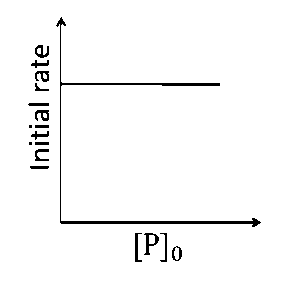
This is first order reaction and for first order reaction $${t_{1/2}} = {{0.693} \over k}$$
So, half-life is independent of initial concentration.
Therefore, the plot (a) correct.
For first order reaction,
$$(r) = k[{(C{H_3})_3}C - Br]$$ ;
$$\ln \left( {{{{P_0}} \over P}} \right) = k\,t$$
or $$\ln \left( {{P \over {{P_0}}}} \right) = - k\,t$$
Hence, plot (b) and (d) are incorrect. For first order reaction,
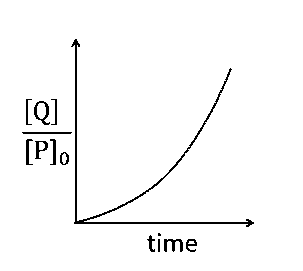
Q = [P0](1 $$-$$ ekt)
Or $${{[Q]} \over {[{P_0}]}} = (1 - {e^{kt}})$$
Hence, plot (c) is incorrect.
This is first order reaction and for first order reaction $${t_{1/2}} = {{0.693} \over k}$$
So, half-life is independent of initial concentration.
Therefore, the plot (a) correct.
For first order reaction,
$$(r) = k[{(C{H_3})_3}C - Br]$$ ;
$$\ln \left( {{{{P_0}} \over P}} \right) = k\,t$$
or $$\ln \left( {{P \over {{P_0}}}} \right) = - k\,t$$
Hence, plot (b) and (d) are incorrect. For first order reaction,
Q = [P0](1 $$-$$ ekt)
Or $${{[Q]} \over {[{P_0}]}} = (1 - {e^{kt}})$$
Hence, plot (c) is incorrect.
Comments (0)


