JEE Advance - Chemistry (2020 - Paper 2 Offline - No. 16)
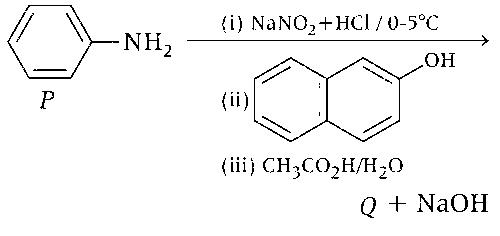
Consider the reaction sequence from P to Q shown below. The overall yield of the major product Q from P is 75%. What is the amount in grams of Q obtained from 9.3 mL of P? (Use density of P = 1.00 g mL-1; Molar mass of C = 12.0, H = 1.0, O = 16.0 and N = 14.0 g mol-1)

Answer
18.6
18.6g
- OR
18.60
- OR
18.60g
- OR
Explanation
Aniline (P) is treated with NaNO2 and HCl in cold condition to form benzene diazonium chloride. The process of conversion of primary aromatic amines into its diazonium salt is called diazotisation.
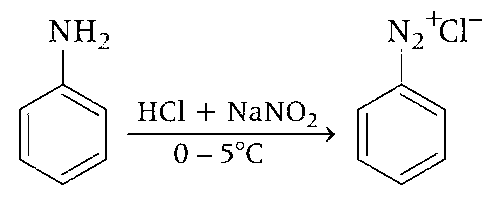
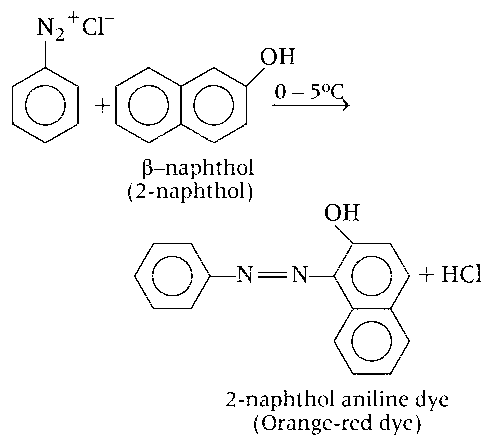
$$\beta $$-naphthol couples with phenyldiazonium electrophile to produce an intense orange-red dye (Q) as major product.
Given that, volume of aniline (P) = 9.3 mL (density of P = 1.00 g/mL)
So, mass of aniline = 9.3 g
Molecular mass of aniline (C6H7N) = 93 g/mol
Therefore, moles of aniline = 9.3 / 93 = 0.1 mol of P.
Molecular mass of 2 napthol aniline orange dye (Q) = 248 g/mol
$$ \Rightarrow $$ 0.1 mol of aniline (P) will produce 0.1 mol of compound (Q).
But, according to the question the major product Q from P is 75%.
Therefore, mass of 'Q' produced
= (0.1 $$ \times $$ 248 $$ \times $$ 0.75)g = 18.60 g
$$\beta $$-naphthol couples with phenyldiazonium electrophile to produce an intense orange-red dye (Q) as major product.

Given that, volume of aniline (P) = 9.3 mL (density of P = 1.00 g/mL)
So, mass of aniline = 9.3 g
Molecular mass of aniline (C6H7N) = 93 g/mol
Therefore, moles of aniline = 9.3 / 93 = 0.1 mol of P.
Molecular mass of 2 napthol aniline orange dye (Q) = 248 g/mol
$$ \Rightarrow $$ 0.1 mol of aniline (P) will produce 0.1 mol of compound (Q).
But, according to the question the major product Q from P is 75%.
Therefore, mass of 'Q' produced
= (0.1 $$ \times $$ 248 $$ \times $$ 0.75)g = 18.60 g
Comments (0)


