JEE Advance - Chemistry (2020 - Paper 2 Offline - No. 5)
The structure of a peptide is given below.
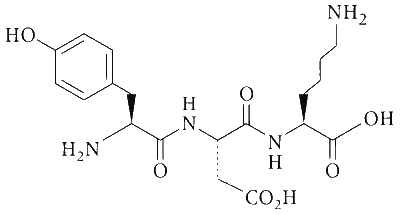
If the absolute values of the net charge of the peptide at $\mathrm{pH}$ $=2$, $\mathrm{pH}=6$, and $\mathrm{pH}=11$ are $\left|Z_1\right|,\left|Z_2\right|$, and $\left|Z_3\right|$, respectively, then what is $\left|Z_1\right|+\left|Z_2\right|+\left|Z_3\right|$?

If the absolute values of the net charge of the peptide at $\mathrm{pH}$ $=2$, $\mathrm{pH}=6$, and $\mathrm{pH}=11$ are $\left|Z_1\right|,\left|Z_2\right|$, and $\left|Z_3\right|$, respectively, then what is $\left|Z_1\right|+\left|Z_2\right|+\left|Z_3\right|$?
Answer
5
Explanation
At pH = 2,
There are two $$-$$NH2 group, and + 1 charge on each group because all amino groups exist in the form of $$-$$NH$$_3^ \oplus $$.
Therefore, |Z1| = 2.
At pH = 6,
NH2 of lysine (+ 1) (pH = 9.47) and COOH ($$-$$1) of glutamic (pH = 3.08) acid, so because of dipolar ion exists, therefore |Z2| = 0.
At pH = 11,
COOH of glutamic acid has ($$-$$1), COOH of lysine ($$-$$1) and OH of phenol ($$-$$1).
Therefore, |Z3| = |$$-$$3| = 3 (All COOH and OH exist in the form of $$-$$COO$$-$$ and $$-$$O$$-$$).
$$ \therefore $$ |Z1| + |Z2| + |Z3| = 2 + 0 + 3 = 5
There are two $$-$$NH2 group, and + 1 charge on each group because all amino groups exist in the form of $$-$$NH$$_3^ \oplus $$.
Therefore, |Z1| = 2.
At pH = 6,
NH2 of lysine (+ 1) (pH = 9.47) and COOH ($$-$$1) of glutamic (pH = 3.08) acid, so because of dipolar ion exists, therefore |Z2| = 0.
At pH = 11,
COOH of glutamic acid has ($$-$$1), COOH of lysine ($$-$$1) and OH of phenol ($$-$$1).
Therefore, |Z3| = |$$-$$3| = 3 (All COOH and OH exist in the form of $$-$$COO$$-$$ and $$-$$O$$-$$).
$$ \therefore $$ |Z1| + |Z2| + |Z3| = 2 + 0 + 3 = 5
Comments (0)


