JEE Advance - Chemistry (2020 - Paper 2 Offline - No. 11)
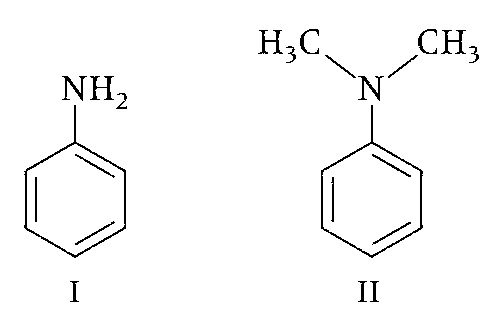
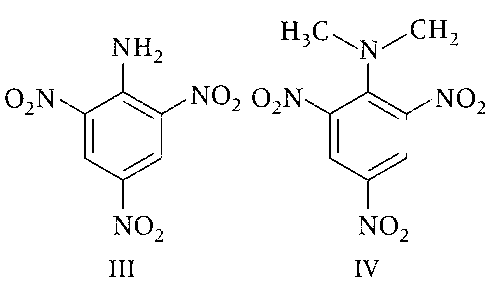
Consider the following four compounds, I, II, III, and IV.
Choose the correct statement(s)


Choose the correct statement(s)
The order of basicity is II > I > III > IV.
The magnitude of pKb difference between I and II is more than that between III and IV.
Resonance effect is more in III than in IV.
Steric effect makes compound IV more basic than III.
Explanation
The correct basic strength order is
(IV) > (II) > (I) > (III);
(IV) is strongest base due to steric inhibition to resonance effect.
(III) is weakest base due to $$-$$M group of three nitro groups present at ortho and para positions.
(II) is stronger than (I) since (III) is tertiary and (I) primary aromatic amine.
So, option (a) is incorrect.
(b) pKb different between I and II is 0.53 and that of III and IV is 4.6. So, option (b) is incorrect.
(c) and (d) In 2, 4, 6-trinitro aniline (III) due to strong $$-$$R effect of $$-$$NO2 groups, the lone pair of $$-$$NH2 is more involved with benzene ring hence it has least basic strength. Whereas (IV) N, N-dimethyl 2, 4, 6-trinitro aniline, due to steric inhibition to resonance (SIR) effect; the lone pair of nitrogen is not in the plane of benzene, hence makes it lone pair more free to protonate.
(IV) > (II) > (I) > (III);
(IV) is strongest base due to steric inhibition to resonance effect.
(III) is weakest base due to $$-$$M group of three nitro groups present at ortho and para positions.
(II) is stronger than (I) since (III) is tertiary and (I) primary aromatic amine.
So, option (a) is incorrect.
(b) pKb different between I and II is 0.53 and that of III and IV is 4.6. So, option (b) is incorrect.
(c) and (d) In 2, 4, 6-trinitro aniline (III) due to strong $$-$$R effect of $$-$$NO2 groups, the lone pair of $$-$$NH2 is more involved with benzene ring hence it has least basic strength. Whereas (IV) N, N-dimethyl 2, 4, 6-trinitro aniline, due to steric inhibition to resonance (SIR) effect; the lone pair of nitrogen is not in the plane of benzene, hence makes it lone pair more free to protonate.
Comments (0)


