JEE Advance - Chemistry (2020 - Paper 2 Offline - No. 7)
In an experiment, m grams of a compound X (gas/liquid/solid) taken in a container is loaded in a balance as shown in figure I below.
In the presence of a magnetic field, the pan with X is either deflected upwards (figure II), or deflected downwards (figure III), depending on the compound X. Identify the correct statement(s).
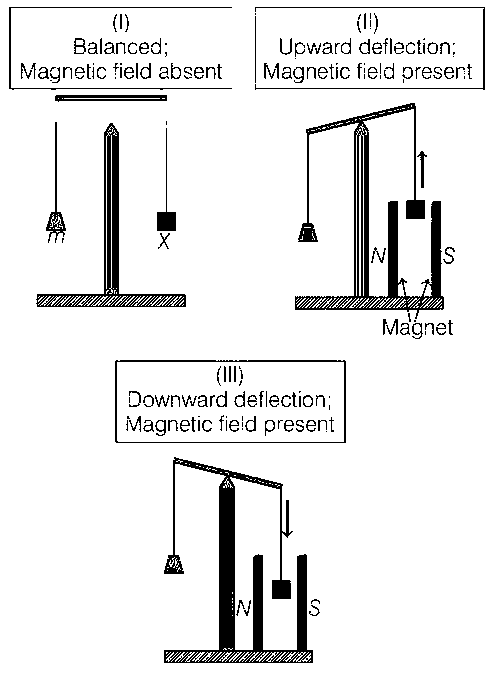
In the presence of a magnetic field, the pan with X is either deflected upwards (figure II), or deflected downwards (figure III), depending on the compound X. Identify the correct statement(s).

If X is H2O(l), deflection of the pan is upwards
If X is K4[Fe(CN)6](s), deflection of the pan is upwards.
If X is O2(g), deflection of the pan is downwards.
If X is C6H6(l), deflection of the pan is downwards
Explanation
Paramagnetism is a form of magnetism whereby some materials are attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. So, magnetic balance shows downward deflection. While diamagnetic substance shows repulsion in magnetic field and magnetic balance shows upward deflection.
(a) X = H2O(l)
(Water has no unpaired electrons and is thus diamagnetic). Hence, statement (a) is correct.
(b) X = K4[Fe(CN)6](s)
(CN is a strong field ligand which forces the d-orbital electrons to pair up ($$t_{2g}^6e_g^0$$) and making it diamagnetic. Hence, statement (b) is correct.
(c) X = O2 (g)
Here, O2(g) is paramagnetic due to two-unpaired electrons present in $$\pi $$* (antibonding orbitals). Hence, statement (c) is correct.
(d) X = C6H6(l)
(Here, C6H6 is diamagnetic due to presence of 0 unpaired electrons. Hence, statement (d) is incorrect.
(a) X = H2O(l)
(Water has no unpaired electrons and is thus diamagnetic). Hence, statement (a) is correct.
(b) X = K4[Fe(CN)6](s)
(CN is a strong field ligand which forces the d-orbital electrons to pair up ($$t_{2g}^6e_g^0$$) and making it diamagnetic. Hence, statement (b) is correct.
(c) X = O2 (g)
Here, O2(g) is paramagnetic due to two-unpaired electrons present in $$\pi $$* (antibonding orbitals). Hence, statement (c) is correct.
(d) X = C6H6(l)
(Here, C6H6 is diamagnetic due to presence of 0 unpaired electrons. Hence, statement (d) is incorrect.
Comments (0)


