JEE Advance - Chemistry (2020 - Paper 2 Offline - No. 6)
An organic compound (C8H10O2) rotates plane-porarised light. It produces pink color with neutral FeCl3 solution. What is the total number of all the possible isomers for this compound?
Answer
6
Explanation
Phenolic ($$-$$OH) group gives positive test with neutral FeCl3 solution, means organic compound (C8H10O2) also rotate plane porarised light means compound is an optically active and chiral carbon atom is present.
So, the possible structures which are optically active and have phenolic group are as followed :
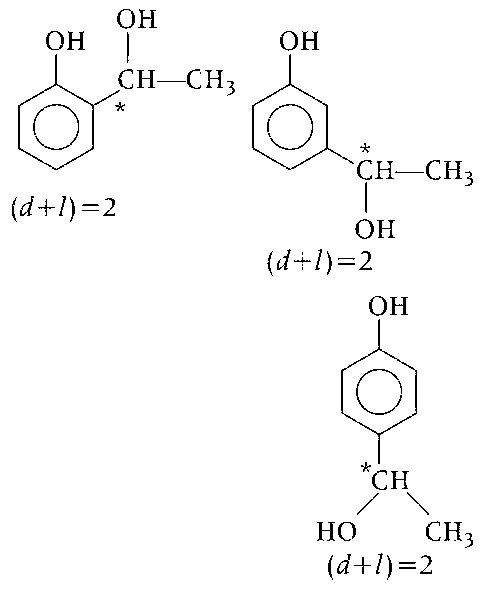
Therefore, total optically active isomers will be 6.
So, the possible structures which are optically active and have phenolic group are as followed :

Therefore, total optically active isomers will be 6.
Comments (0)


