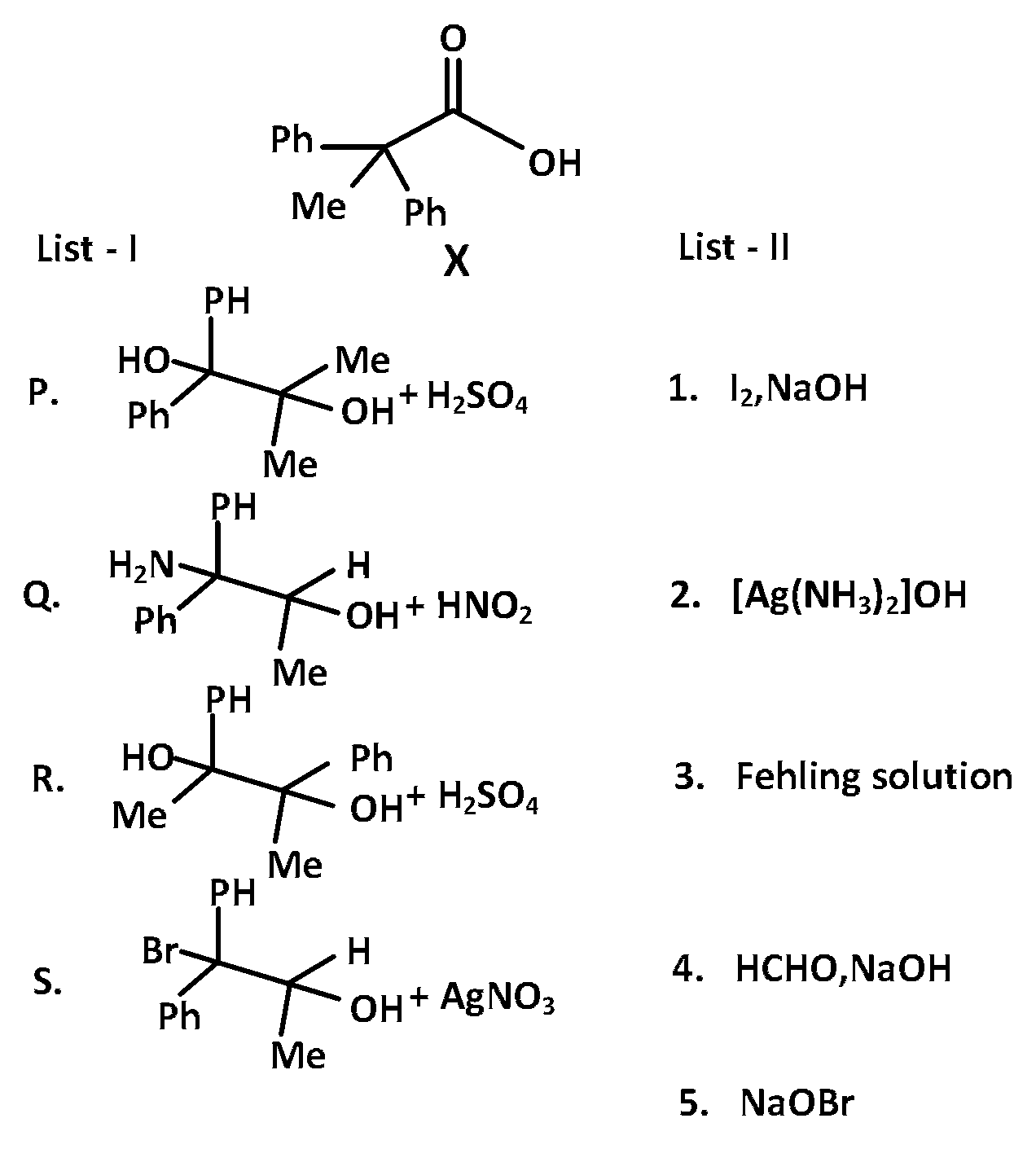
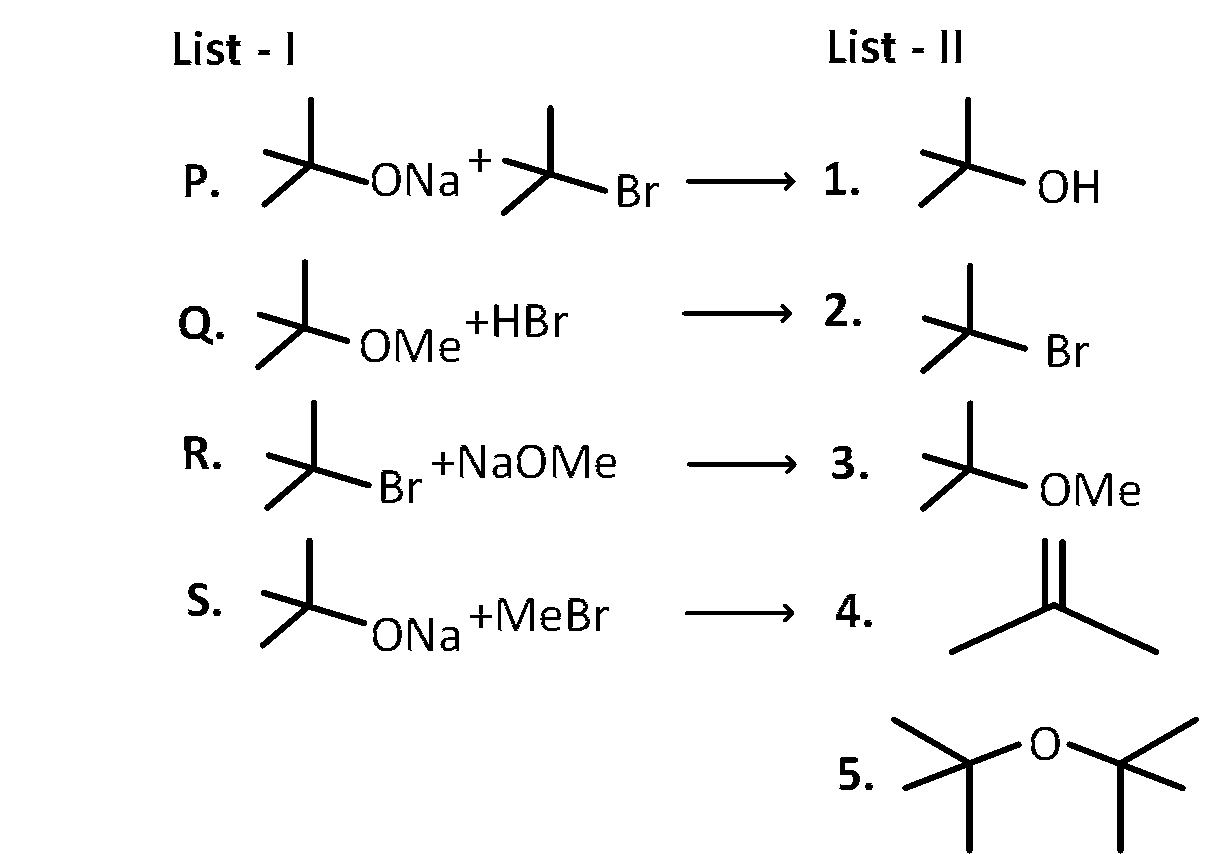
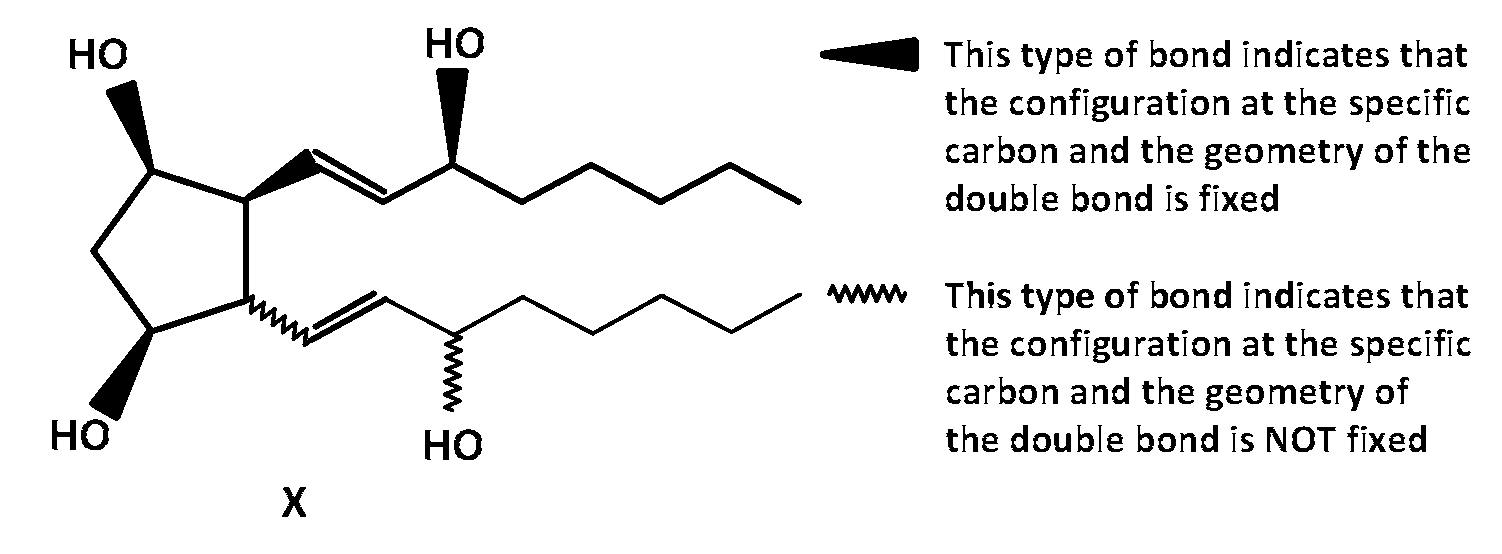
JEE Advance - Chemistry (2018 - Paper 2 Offline)
2
For a reaction, $$A\,\,\rightleftharpoons\,\,P,$$ the plots of $$\left[ A \right]$$ and $$\left[ P \right]$$ with time at temperature $${T_1}$$ and $${T_2}$$ are given below.
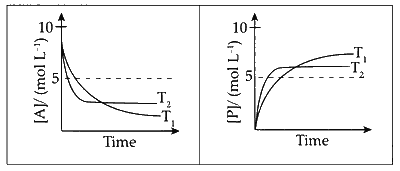
If $${T_2} > {T_1},$$ the correct statement(s) is (are) (Assume $$\Delta {H^ \circ }$$ and $$\Delta {S^ \circ }$$ are independent of temperature and ratio of $$lnK$$ at $${T_1}$$ to $$lnK$$ at $${T_2}$$ is greater than $${{{T_2}} \over {{T_1}}}.$$ Here $$H,$$ $$S,G$$ and $$K$$ are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)

If $${T_2} > {T_1},$$ the correct statement(s) is (are) (Assume $$\Delta {H^ \circ }$$ and $$\Delta {S^ \circ }$$ are independent of temperature and ratio of $$lnK$$ at $${T_1}$$ to $$lnK$$ at $${T_2}$$ is greater than $${{{T_2}} \over {{T_1}}}.$$ Here $$H,$$ $$S,G$$ and $$K$$ are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)
Answer
A
C
3
Galena (an ore) is partially oxidized by passing air through it at high temperature. After some time, the passage of air is stopped, but the heating is continued in a closed furnace such that the contents undergo self-reduction. The weight (in kg) of $$Pb$$ produced per kg of $${O_2}$$ consumed is ___________.
(Atomic weights in $$g\,mo{l^{ - 1}}:O = 16,S = 32,Pb = 207$$)
(Atomic weights in $$g\,mo{l^{ - 1}}:O = 16,S = 32,Pb = 207$$)
Answer
6.47
6
To measure the quantity of $$MnC{l_2}$$ dissolved in an aqueous solution, it was completely converted to $$KMn{O_4}$$ using the reaction,
$$MnC{l_2} + {K_2}{S_2}{O_8} + {H_2}O \to KMn{O_4} + {H_2}S{O_4} + HCl$$ (equation not balanced).
Few drops of concentrated $$HCl$$ were added to this solution and gently warmed. Further, oxalic acid ($$225$$ $$mg$$) was added in portions till the colour of the permanganate ion disappeared. The quantity of $$MnC{l_2}$$ (in mg) present in the initial solution is ____________.
(Atomic weights in $$g\,\,mo{l^{ - 1}}:Mn = 55,Cl = 35.5$$ )
$$MnC{l_2} + {K_2}{S_2}{O_8} + {H_2}O \to KMn{O_4} + {H_2}S{O_4} + HCl$$ (equation not balanced).
Few drops of concentrated $$HCl$$ were added to this solution and gently warmed. Further, oxalic acid ($$225$$ $$mg$$) was added in portions till the colour of the permanganate ion disappeared. The quantity of $$MnC{l_2}$$ (in mg) present in the initial solution is ____________.
(Atomic weights in $$g\,\,mo{l^{ - 1}}:Mn = 55,Cl = 35.5$$ )
Answer
126
7
The surface of copper gets tarnished by the formation of copper oxide. $${N_2}$$ gas was passed to prevent the oxide formation during heating of copper at $$1250$$ $$K.$$ However, the $${N_2}$$ gas contains $$1$$ mole % of water vapor as impurity. The water vapor oxidises copper as per the reaction given below : $$2Cu\left( s \right) + {H_2}O\left( g \right) \to C{u_2}O\left( s \right) + {H_2}\left( g \right)$$
$${P_{H2}}$$ is the minimum partial pressure of $${H_2}$$ (in bar) needed to prevent the oxidation at $$1250$$ $$K.$$ The value of $$\ln \left( {{P_{H2}}} \right)$$ is ________.
Given: total pressure $$=1$$ bar, $$R$$ (universal gas constant ) $$=$$ $$8J{K^{ - 1}}\,\,mo{l^{ - 1}},$$ $$\ln \left( {10} \right) = 2.3.\,$$ $$Cu(s)$$ and $$C{u_2}O\left( s \right)$$ are naturally immiscible.
At $$1250$$ $$K:2Cu(s)$$ $$ + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to C{u_2}O\left( s \right);$$ $$\Delta {G^ \circ } = - 78,000J\,mo{l^{ - 1}}$$
$${H_2}\left( g \right) + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to {H_2}O\left( g \right);$$
$$\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$$ $$\Delta {G^ \circ } = - 1,78,000J\,mo{l^{ - 1}};$$ ($$G$$ is the Gibbs energy)
$${P_{H2}}$$ is the minimum partial pressure of $${H_2}$$ (in bar) needed to prevent the oxidation at $$1250$$ $$K.$$ The value of $$\ln \left( {{P_{H2}}} \right)$$ is ________.
Given: total pressure $$=1$$ bar, $$R$$ (universal gas constant ) $$=$$ $$8J{K^{ - 1}}\,\,mo{l^{ - 1}},$$ $$\ln \left( {10} \right) = 2.3.\,$$ $$Cu(s)$$ and $$C{u_2}O\left( s \right)$$ are naturally immiscible.
At $$1250$$ $$K:2Cu(s)$$ $$ + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to C{u_2}O\left( s \right);$$ $$\Delta {G^ \circ } = - 78,000J\,mo{l^{ - 1}}$$
$${H_2}\left( g \right) + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to {H_2}O\left( g \right);$$
$$\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$$ $$\Delta {G^ \circ } = - 1,78,000J\,mo{l^{ - 1}};$$ ($$G$$ is the Gibbs energy)
Answer
-14.6
9
In the following reaction sequence, the amount of $$D$$ (in g) formed from $$10$$ moles of acetophenone is ___________.
(Atomic weights in $$g\,mo{l^{ - 1}}:H = 1,C = 12,$$ $$N = 14,O = 16,$$ $$Br = 80.$$. The yield (%) corresponding to the product in each step is given in the parenthesis)
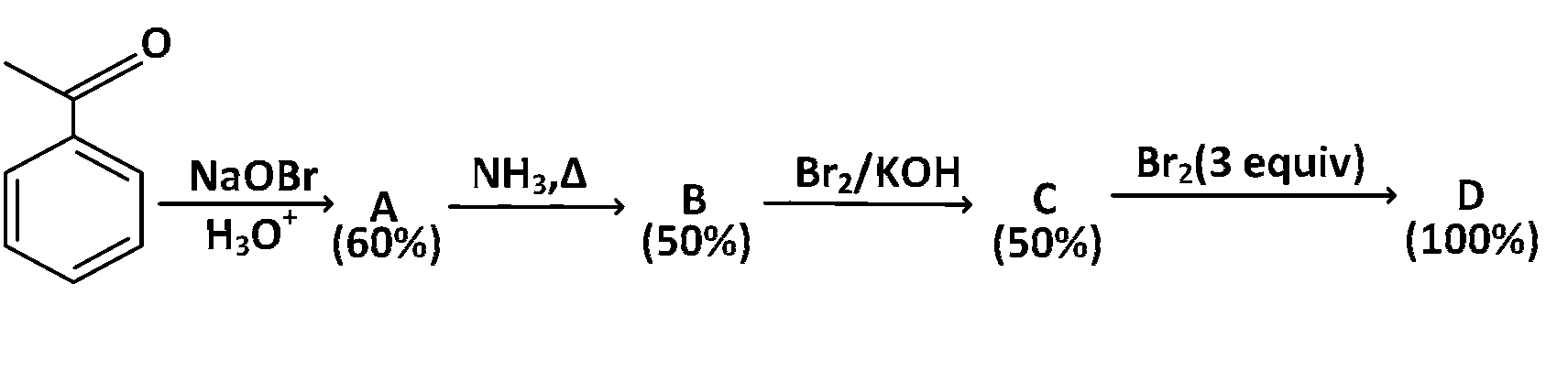
(Atomic weights in $$g\,mo{l^{ - 1}}:H = 1,C = 12,$$ $$N = 14,O = 16,$$ $$Br = 80.$$. The yield (%) corresponding to the product in each step is given in the parenthesis)

Answer
495
Or +1 more10
For a first order reaction $$A\left( g \right) \to 2B\left( g \right) + C\left( g \right)$$ at constant volume and $$300K,$$ the total pressure at the beginning $$(t=0)$$ and at time $$t$$ are $${P_0}$$ and $${P_1},$$ respectively. Initially, only $$A$$ is present with concentration $${\left[ A \right]_0},$$ and $${t_{1/3}}$$ is the time required for the partial pressure of $$A$$ to reach $$1/{3^{rd}}$$ of its initial value. The correct option(s) is (are) (Assume that all these gases behave as ideal gases)
Answer
A
D
11
Consider an electrochemical cell :
$$A\left( s \right)\left| {{A^{n + }}\left( {aq,2M} \right)} \right|{B^{2n + }}\left( {aq,1M} \right)\left| {B\left( s \right).} \right.$$
The value of $$\Delta {H^ \circ }$$ for the cell reaction is twice that of $$\Delta {G^ \circ }$$ at $$300$$ $$K.$$ If the $$emf$$ of the cell is zero, the $$\Delta {S^ \circ }$$ (in $$J\,{K^{ - 1}}mo{l^{ - 1}}$$) of the cell reaction per mole of $$B$$ formed at $$300$$ $$K$$ is ___________.
(Given: $$\ln \left( 2 \right) = 0.7,R$$ (universal gas constant) $$ = 8.3J\,{K^{ - 1}}\,mo{l^{ - 1}}.$$ $$H,S$$ and $$G$$ are enthalpy, entropy and Gibbs energy, respectively.)
$$A\left( s \right)\left| {{A^{n + }}\left( {aq,2M} \right)} \right|{B^{2n + }}\left( {aq,1M} \right)\left| {B\left( s \right).} \right.$$
The value of $$\Delta {H^ \circ }$$ for the cell reaction is twice that of $$\Delta {G^ \circ }$$ at $$300$$ $$K.$$ If the $$emf$$ of the cell is zero, the $$\Delta {S^ \circ }$$ (in $$J\,{K^{ - 1}}mo{l^{ - 1}}$$) of the cell reaction per mole of $$B$$ formed at $$300$$ $$K$$ is ___________.
(Given: $$\ln \left( 2 \right) = 0.7,R$$ (universal gas constant) $$ = 8.3J\,{K^{ - 1}}\,mo{l^{ - 1}}.$$ $$H,S$$ and $$G$$ are enthalpy, entropy and Gibbs energy, respectively.)
Answer
-11.62
12
Consider the following reversible reaction, $$A\left( g \right) + B\left( g \right) \to AB\left( g \right).$$
The activation energy of the backward reaction exceeds that of the forward reaction by $$2RT$$ (in $$J\,mo{l^{ - 1}}$$). If the pre-exponential factor of the forward reaction is $$4$$ times that of the reverse reaction, the absolute value of $$\Delta {G^ \circ }$$ (in $$J\,mo{l^{ - 1}}$$ ) for the reaction at $$300$$ $$K$$ is ____________.
(Given; $$\ln \left( 2 \right) = 0.7,RT = 2500$$ $$J\,mo{l^{ - 1}}$$ at $$300$$ $$K$$ and $$G$$ is the Gibbs energy)
The activation energy of the backward reaction exceeds that of the forward reaction by $$2RT$$ (in $$J\,mo{l^{ - 1}}$$). If the pre-exponential factor of the forward reaction is $$4$$ times that of the reverse reaction, the absolute value of $$\Delta {G^ \circ }$$ (in $$J\,mo{l^{ - 1}}$$ ) for the reaction at $$300$$ $$K$$ is ____________.
(Given; $$\ln \left( 2 \right) = 0.7,RT = 2500$$ $$J\,mo{l^{ - 1}}$$ at $$300$$ $$K$$ and $$G$$ is the Gibbs energy)
Answer
8500
13
Consider the following reversible reaction, $$A\left( g \right) + B\left( g \right) \to AB\left( g \right).$$
The activation energy of the backward reaction exceeds that of the forward reaction by $$2RT$$ (in $$J\,mo{l^{ - 1}}$$). If the pre-exponential factor of the forward reaction is $$4$$ times that of the reverse reaction, the absolute value of $$\Delta {G^ \circ }$$ (in $$J\,mo{l^{ - 1}}$$ ) for the reaction at $$300$$ $$K$$ is ____________.
(Given; $$\ln \left( 2 \right) = 0.7,RT = 2500$$ $$J\,mo{l^{ - 1}}$$ at $$300$$ $$K$$ and $$G$$ is the Gibbs energy)
The activation energy of the backward reaction exceeds that of the forward reaction by $$2RT$$ (in $$J\,mo{l^{ - 1}}$$). If the pre-exponential factor of the forward reaction is $$4$$ times that of the reverse reaction, the absolute value of $$\Delta {G^ \circ }$$ (in $$J\,mo{l^{ - 1}}$$ ) for the reaction at $$300$$ $$K$$ is ____________.
(Given; $$\ln \left( 2 \right) = 0.7,RT = 2500$$ $$J\,mo{l^{ - 1}}$$ at $$300$$ $$K$$ and $$G$$ is the Gibbs energy)
Answer
8500
14
Consider an electrochemical cell :
$$A\left( s \right)\left| {{A^{n + }}\left( {aq,2M} \right)} \right|{B^{2n + }}\left( {aq,1M} \right)\left| {B\left( s \right).} \right.$$
The value of $$\Delta {H^ \circ }$$ for the cell reaction is twice that of $$\Delta {G^ \circ }$$ at $$300$$ $$K.$$ If the $$emf$$ of the cell is zero, the $$\Delta {S^ \circ }$$ (in $$J\,{K^{ - 1}}mo{l^{ - 1}}$$) of the cell reaction per mole of $$B$$ formed at $$300$$ $$K$$ is ___________.
(Given: $$\ln \left( 2 \right) = 0.7,R$$ (universal gas constant) $$ = 8.3J\,{K^{ - 1}}\,mo{l^{ - 1}}.$$ $$H,S$$ and $$G$$ are enthalpy, entropy and Gibbs energy, respectively.)
$$A\left( s \right)\left| {{A^{n + }}\left( {aq,2M} \right)} \right|{B^{2n + }}\left( {aq,1M} \right)\left| {B\left( s \right).} \right.$$
The value of $$\Delta {H^ \circ }$$ for the cell reaction is twice that of $$\Delta {G^ \circ }$$ at $$300$$ $$K.$$ If the $$emf$$ of the cell is zero, the $$\Delta {S^ \circ }$$ (in $$J\,{K^{ - 1}}mo{l^{ - 1}}$$) of the cell reaction per mole of $$B$$ formed at $$300$$ $$K$$ is ___________.
(Given: $$\ln \left( 2 \right) = 0.7,R$$ (universal gas constant) $$ = 8.3J\,{K^{ - 1}}\,mo{l^{ - 1}}.$$ $$H,S$$ and $$G$$ are enthalpy, entropy and Gibbs energy, respectively.)
Answer
-11.62
15
Dilution processes of different aqueous solutions, with water, are given in LIST - I. The effects of dilution of the solutions on $$\left[ {{H^ + }} \right]$$ are given in LIST - II
(Note: Degree of dissociation (a) of weak acid and weak base is $$<<1;$$ degree of hydrolysis of salt $$<<1;$$ $$\left[ {{H^ + }} \right]$$ represents the concentration of $${H^ + }$$ ions)
Match each process given in LIST-I with one or more effect(s) in LIST-II. The correct option is :
(Note: Degree of dissociation (a) of weak acid and weak base is $$<<1;$$ degree of hydrolysis of salt $$<<1;$$ $$\left[ {{H^ + }} \right]$$ represents the concentration of $${H^ + }$$ ions)
| LIST-I | LIST-II | ||
|---|---|---|---|
| P. | (10 mL of 0.1 M NaOH + 20 mL of 0.1 M acetic acid) diluted to 60 mL |
1. | the value of [H+] does not change on dilution |
| Q. | (20 mL of 0.1 M NaOH + 20 mL of 0.1 M acetic acid) diluted to 80 mL |
2. | the value of [H+] changes to half of its initial value on dilution |
| R. | (20 mL of 0.1 M HCL + 20 mL of 0.1 M ammonia solution) diluted to 80 mL |
3. | the value of [H+] changes to two times of its initial value on dilution |
| S. | 10 mL saturated solution of Ni(OH)2 in equilibrium with excess solid Ni(OH)2 is diluted to 20 mL (solid Ni(OH)2 is still present after dilution). |
4. | the value of [H+] changes to $${1 \over {\sqrt 2 }}$$ times of its initial value on dilution |
| 5. | the value of [H+] changes to $$\sqrt 2 $$ times of its initial value on dilution |
Match each process given in LIST-I with one or more effect(s) in LIST-II. The correct option is :
Answer
(D)
$$P - 1;Q - 5;R - 4;S - 1$$