JEE Advance - Chemistry (2018 - Paper 2 Offline - No. 5)
The correct option(s) regarding the complex
$${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_3}\left( {{H_2}O} \right)} \right]^{3 + }}\,\,$$ $$\left( {en = {H_2}NC{H_2}C{H_2}N{H_2}} \right)$$ is (are)
$${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_3}\left( {{H_2}O} \right)} \right]^{3 + }}\,\,$$ $$\left( {en = {H_2}NC{H_2}C{H_2}N{H_2}} \right)$$ is (are)
It has two geometrical isomers
It will have three geometrical isomers if bidentate 'en' is replaced by two cyanide ligands
It is paramagnetic
It absorbs light at longer wavelength as compared to
$${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_4}} \right]^{3 + }}$$
$${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_4}} \right]^{3 + }}$$
Explanation
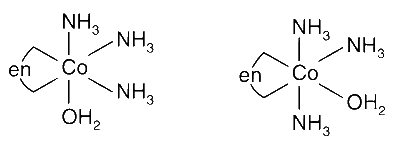
Option (A) : Correct. The complex [Co(en)(NH3)3(H2O)]3+ has two following isomers:
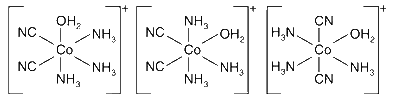
Option (B) : Correct. When bidentate 'en' is replaced by two cyanide ligands complex [Co(CN)2(NH3)3(H2O)]+ will have three following isomers:
Option (C) : Incorrect. Incomplex [Co(en)(NH3)3(H2O)]3+, Co is in + 3 oxidation, that is, [Ar]d6. In the presence given ligands the complex will have low spin, hence, diamagnetic.
Option (D) : Correct. In complex [Co(en)(NH3)4]3+ there is larger gap between t2g and eg than in complex [Co(en)(NH3)3(H2O)]3+. Hence [Co(en)(NH3)3(H2O)]3+ will absorb light at longer wavelength as compared to [Co(en)(NH3)4]3+.
Comments (0)


