JEE Advance - Chemistry (2018 - Paper 2 Offline - No. 17)
LIST-I contains reactions and LIST-II contains major products.
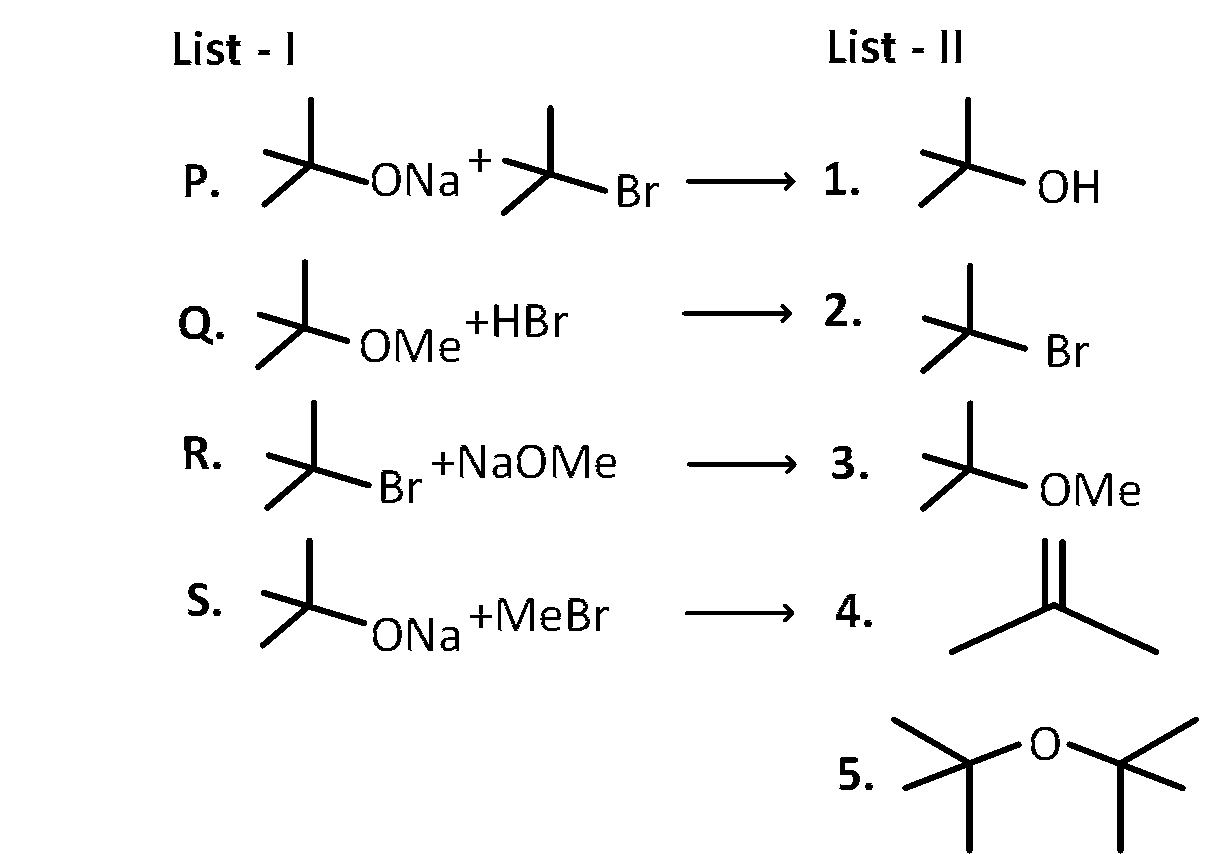
Match the reaction in LIST-I with one or more products in LIST-II and choose the correct option.

Match the reaction in LIST-I with one or more products in LIST-II and choose the correct option.
$$P - 1,5;Q - 2;R - 3;S - 4$$
$$P - 1,4;Q - 2;R - 4;S - 3$$
$$P - 1,4;Q - 1,2;R - 3,4;S - 4$$
$$P - 4,5;Q - 4;R - 4;S - 3,4$$
Explanation
P. Reactant 1 , acts as a base and abstracts hydrogen from $\beta$ carbon of tert butyl bromide halide (reactant 2 ) forming an alkene and an alcohol.
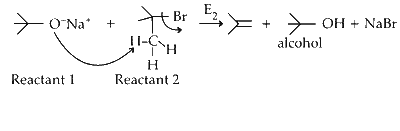
Elimination proceeds via $\mathrm{E}_2$ mechanism. Substitution of reactant 1 is not possible on reactant 2 due to steric crowding at 2.
Q. Reactant 1 (tert butoxide) acts as a base and abstracts proton from acid $(\mathrm{HBr})$ forming an alcohol and methyl bromide.
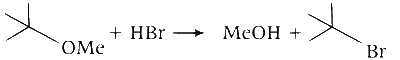
R. Sodium methoxide ( $\mathrm{NaOMe})$ is a strong base and abstracts proton from $\beta$ carbon of tert Butyl with simultaneous less of bromide ion forming an alkene.
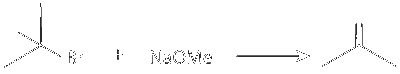
S. Reactant 1 (tert-butoxide) acts as a nucleophile and attacks from the backside of methyl bromide, with subsequent removal of bromide ion. This is an $\mathrm{S}_{\mathrm{N}} 2$ mechanism.


Elimination proceeds via $\mathrm{E}_2$ mechanism. Substitution of reactant 1 is not possible on reactant 2 due to steric crowding at 2.
Q. Reactant 1 (tert butoxide) acts as a base and abstracts proton from acid $(\mathrm{HBr})$ forming an alcohol and methyl bromide.
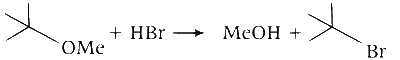
R. Sodium methoxide ( $\mathrm{NaOMe})$ is a strong base and abstracts proton from $\beta$ carbon of tert Butyl with simultaneous less of bromide ion forming an alkene.
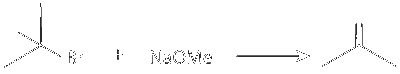
S. Reactant 1 (tert-butoxide) acts as a nucleophile and attacks from the backside of methyl bromide, with subsequent removal of bromide ion. This is an $\mathrm{S}_{\mathrm{N}} 2$ mechanism.

Comments (0)


