JEE Advance - Chemistry (2018 - Paper 2 Offline - No. 6)
To measure the quantity of $$MnC{l_2}$$ dissolved in an aqueous solution, it was completely converted to $$KMn{O_4}$$ using the reaction,
$$MnC{l_2} + {K_2}{S_2}{O_8} + {H_2}O \to KMn{O_4} + {H_2}S{O_4} + HCl$$ (equation not balanced).
Few drops of concentrated $$HCl$$ were added to this solution and gently warmed. Further, oxalic acid ($$225$$ $$mg$$) was added in portions till the colour of the permanganate ion disappeared. The quantity of $$MnC{l_2}$$ (in mg) present in the initial solution is ____________.
(Atomic weights in $$g\,\,mo{l^{ - 1}}:Mn = 55,Cl = 35.5$$ )
$$MnC{l_2} + {K_2}{S_2}{O_8} + {H_2}O \to KMn{O_4} + {H_2}S{O_4} + HCl$$ (equation not balanced).
Few drops of concentrated $$HCl$$ were added to this solution and gently warmed. Further, oxalic acid ($$225$$ $$mg$$) was added in portions till the colour of the permanganate ion disappeared. The quantity of $$MnC{l_2}$$ (in mg) present in the initial solution is ____________.
(Atomic weights in $$g\,\,mo{l^{ - 1}}:Mn = 55,Cl = 35.5$$ )
Answer
126
Explanation
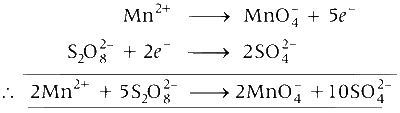
Also,
$$2MnO_4^ - + 5{C_2}O_4^{2 - } \to 2M{n^{2 + }} + 10C{O_2}$$
Hence, $$2M{n^{2 + }} \equiv 5{C_2}O_4^{2 - }$$
$$1MnC{l_2} \equiv 2.5{H_2}{C_2}{O_4}$$
Oxalic acid taken = 225 mg
$$ = {{225} \over {90}} = 2.5$$ millimoles
Hence, MnCl2 = 1 millimole
= (55 + 71) = 126 mg
Comments (0)


