JEE Advance - Chemistry (2018 - Paper 2 Offline - No. 9)
In the following reaction sequence, the amount of $$D$$ (in g) formed from $$10$$ moles of acetophenone is ___________.
(Atomic weights in $$g\,mo{l^{ - 1}}:H = 1,C = 12,$$ $$N = 14,O = 16,$$ $$Br = 80.$$. The yield (%) corresponding to the product in each step is given in the parenthesis)
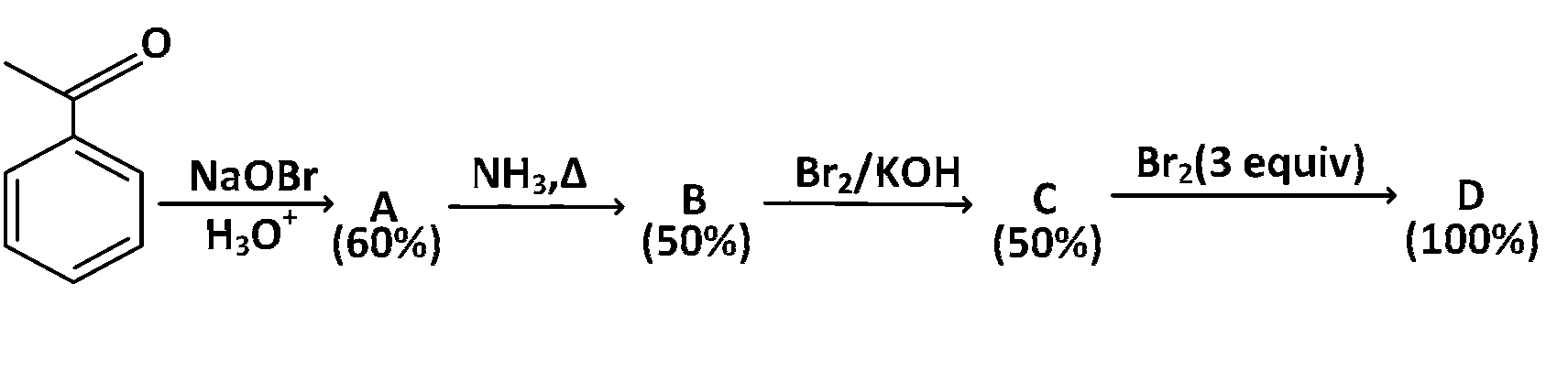
(Atomic weights in $$g\,mo{l^{ - 1}}:H = 1,C = 12,$$ $$N = 14,O = 16,$$ $$Br = 80.$$. The yield (%) corresponding to the product in each step is given in the parenthesis)

Answer
495
495g
- OR
Explanation
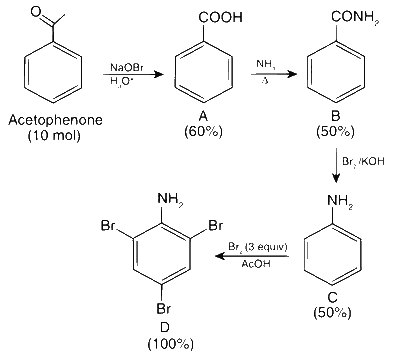
The amount of D formed in mol is $${{60} \over {100}} \times {{50} \over {100}} \times {{50} \over {100}} \times 10 = 1.5$$ mol
Amount of D in grams is = 1.5 $$\times$$ 330 g = 495 g
Comments (0)


