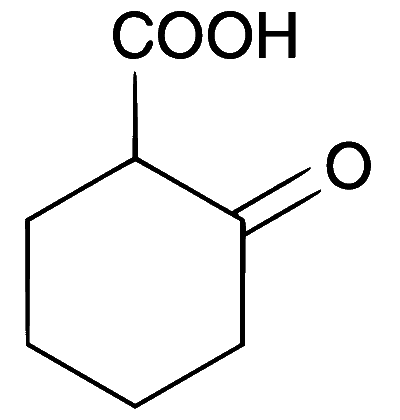
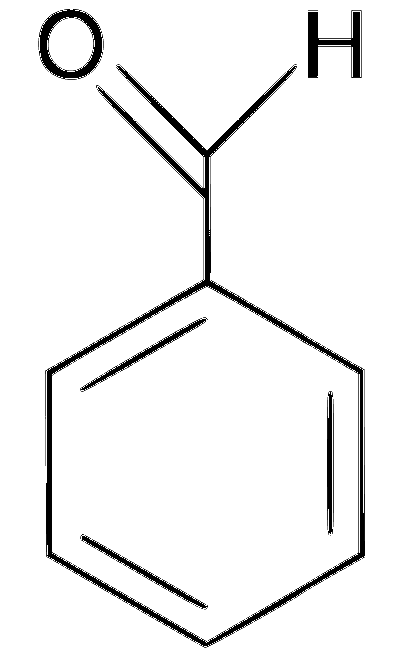
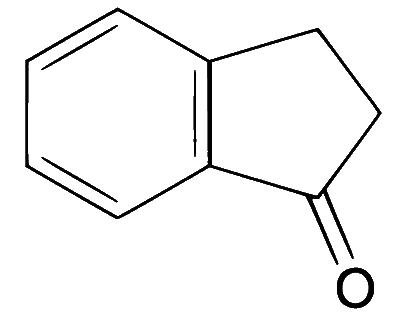
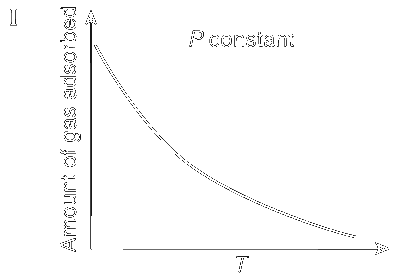
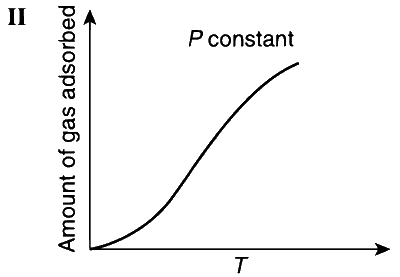
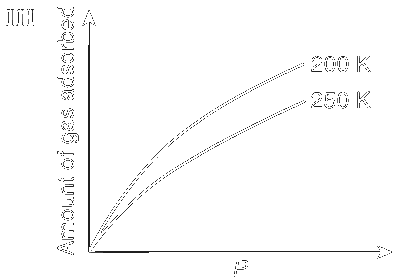
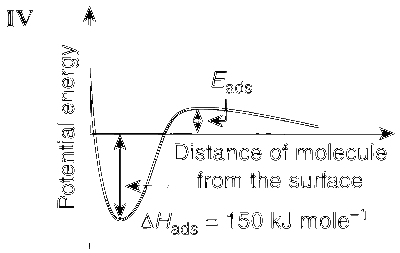
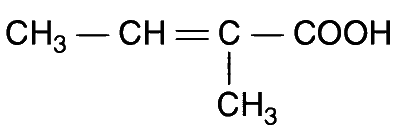JEE Advance - Chemistry (2012 - Paper 2 Offline)
2
The electrochemical cell shown below is a concentration cell. M | M2+ (saturated solution of a sparingly soluble salt, MX2) || M2+ (0.001 mol dm–3) | M The emf of the cell depends on the difference in concentrations of M2+ ions at the two electrodes. The emf of the cell at 298 K is 0.059 V.
The value of ∆G (kJ mol–1) for the given cell is (take 1F = 96500 C mol–1)
The value of ∆G (kJ mol–1) for the given cell is (take 1F = 96500 C mol–1)
Answer
(D)
-11.4
3
The electrochemical cell shown below is a concentration cell. M | M2+ (saturated solution of a sparingly soluble salt, MX2) || M2+ (0.001 mol dm–3) | M The emf of the cell depends on the difference in concentrations of M2+ ions at the two electrodes. The emf of the cell at 298 K is 0.059 V.
The solubility product (Ksp; mol3 dm–9) of MX2 at 298 K based on the information available for the given concentration cell is (take 2.303 $$\times$$ R $$\times$$ 298/F = 0.059 V)
The solubility product (Ksp; mol3 dm–9) of MX2 at 298 K based on the information available for the given concentration cell is (take 2.303 $$\times$$ R $$\times$$ 298/F = 0.059 V)
Answer
(B)
4 $$\times$$ 10–15
4
For a dilute solution containing 2.5 g of a non-volatile non-electrolyte solute in 100 g of water. the elevation in boiling point at 1 atm pressure is 2oC. Assuming concentration of solute is much lower than the concentration of solvent, the vapour pressure (mm of Hg) of the solution is (take Kb = 0.76 K Kg mol-1)
Answer
(A)
724
10
Using the data provided, calculate the multiple bond energy (kJ mol$$-$$1) of a C=C bond in C2H2. That energy is (take the bond energy of C-H bond as 350 kJ mol$$-$$1).
$$\matrix{ \hfill {2C(s) + {H_2}(g) \to {C_2}{H_2}} & \hfill {\Delta H = 225\,kJ\,mo{l^{ - 1}}} \cr \hfill {2C(s) \to 2C(g)} & \hfill {\Delta H = 1410\,kJ\,mo{l^{ - 1}}} \cr \hfill {{H_2}(g) \to 2H(g)} & \hfill {\Delta H = 330\,kJ\,mo{l^{ - 1}}} \cr } $$
Answer
(D)
815 kJ mol$$-$$1