JEE Advance - Chemistry (2012 - Paper 2 Offline - No. 15)
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is(are) correct?
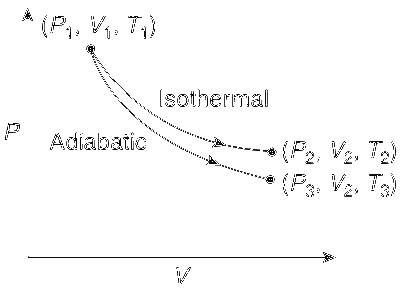
T1 = T2
T3 > T1
wisothermal > wadiabatic
$$\Delta$$Uisothermal > $$\Delta$$Uadiabatic
Explanation
For isothermal process T1 = T2. Work done in isothermal process is less than adiabatic process. In case of isothermal process, the temperature remains constant so there is no change in the internal energy, whereas in case of adiabatic process expansion occurs through internal energy.
Comments (0)


