JEE Advance - Chemistry (2012 - Paper 2 Offline - No. 20)
The given graphs/data I, II, III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure. Which of the following choice(s) about I, II, III and IV is(are) correct?
Explanation
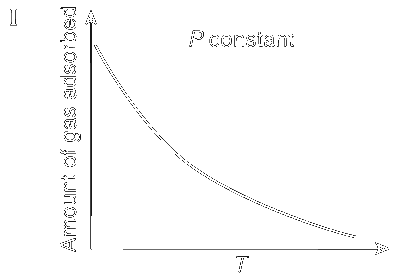
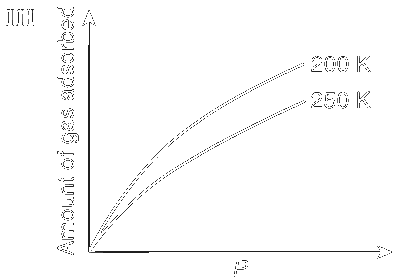
In the case of physiorption, with the increase of temperature and pressure the rate of adsorption decreases because according to Le Chatelier's principle, increase of temperature and pressure will shift the equilibrium to the left
Adsorbate + Adsorbent $$\rightleftharpoons$$ Adsorption + Heat
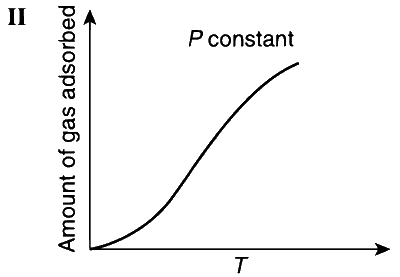
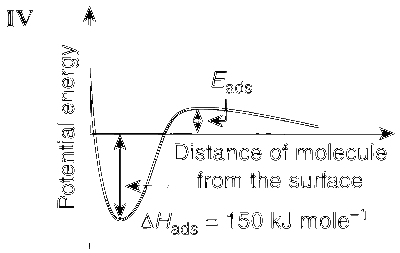
This is shown in Graphs I and III; whereas in the case of chemisorption, there is a formation of strong bond between the adsorbate and the adsorbent and so the rate of adsorption increases with increase in temperature (Graphs II and IV).
Comments (0)


