JEE Advance - Chemistry (2012 - Paper 2 Offline - No. 16)
For the given aqueous reactions, which of the statement(s) is(are) true?
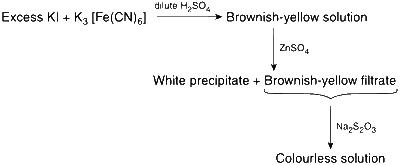
The first reaction is a redox reaction.
White precipitate is Zn3[Fe(CN)6]2.
Addition of filtrate to starch solution gives blue colour.
White precipitate is soluble in NaOH solution.
Explanation
The first reaction is
$${K_3}[Fe{(CN)_6}] + \mathop {KI}\limits_{excess} \to {K_4}[Fe{(CN)_6}] + \mathop {K{I_3}}\limits_{brownish\,yellow\,solution} $$
This is a redox reaction as both oxidation and reduction are taking place.
$$\mathop {{K_4}[Fe{{(CN)}_6}]}\limits_{soluble} \, + ZnS{O_4} \to \mathop {{K_2}Z{n_3}{{[Fe{{(CN)}_6}]}_3}}\limits_{white\,ppt} + NaOH \to \mathop {N{a_2}{{[Zn{{(OH)}_4}]}^{2 - }}}\limits_{soluble} $$
$$2I_3^ - + 2N{a_2}{S_2}{O_3} \to \mathop {N{a_2}{S_4}{O_6}}\limits_{clear\,solution} + 2NaI + \mathop {2{I_2}}\limits_{(blue\,colour)} $$
Comments (0)


