JEE Advance - Chemistry (2012 - Paper 2 Offline - No. 8)
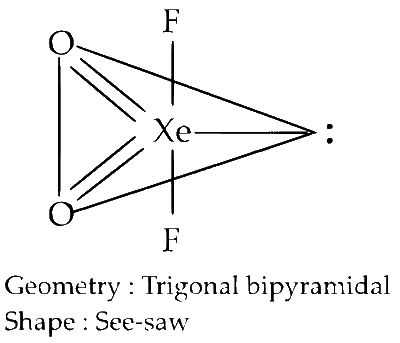
The shape of XeO2F2 molecule is
trigonal bipyramidal.
square planar.
tetrahedral.
see-saw.
Explanation
The electronic configuration of Xe is 5s2 5p6; and that of Xe in excited state is 5s2 5p5 5d1. The hybridisation is sp3d and geometry is see-saw. The actual shape is trigonal bipyramidal but due to the preaence of lone pair it gets distorted to see-saw structure.
Comments (0)


