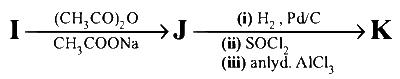JEE Advance - Chemistry (2012 - Paper 2 Offline - No. 11)
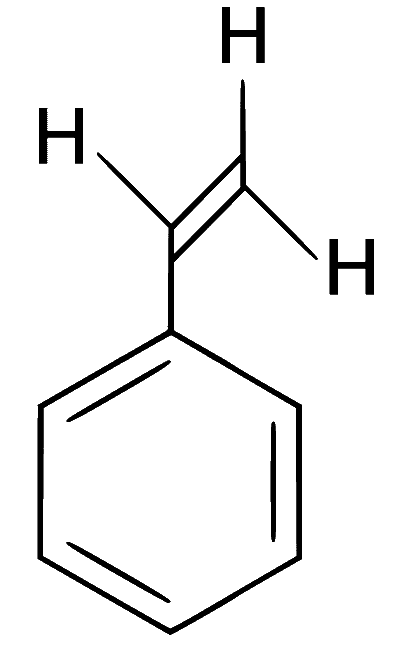
The compound I is
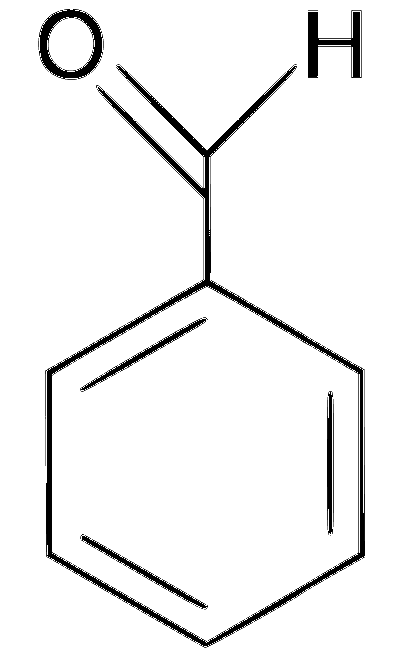
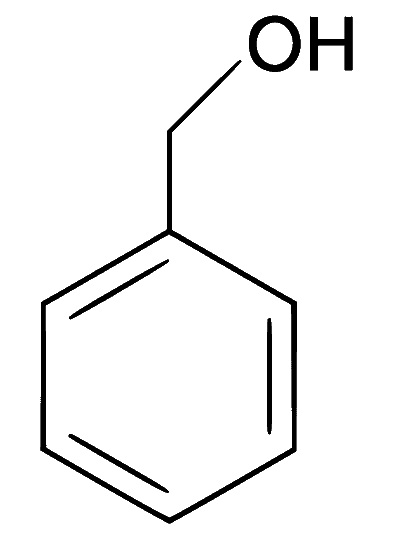
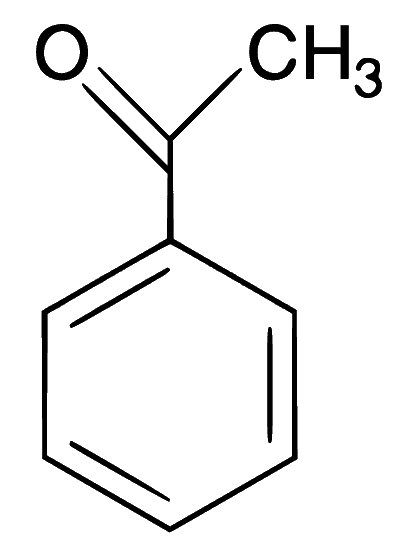
Explanation
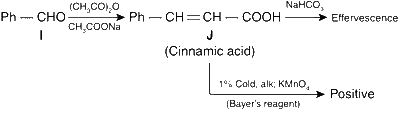
In the given reaction, an aromatic aldehyde is condensed with an acid anhydride (Perkin condensation) in the presence of a base to form $$\alpha$$,$$\beta$$-unsaturated acid (cinnamic acid). This gives effervescence on reaction with NaHCO3 and positive test with Bayer's reagent (1% alkaline KMnO4).
Comments (0)