JEE Advance - Chemistry (2012 - Paper 2 Offline - No. 6)
$$NiC{l_2}{\{ P{({C_2}{H_5})_2}({C_6}{H_5})\} _2}$$ exhibits temperature-dependent magnetic behaviour (paramagnetic/diamagnetic). The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are, respectively,
tetrahedral and tetrahedral.
square planar and square planar.
tetrahedral and square planar.
square planar and tetrahedral.
Explanation
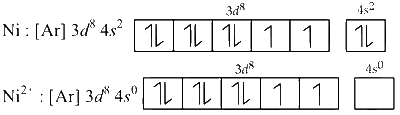
The configuration of Ni = 3d8 4s2 and that of Ni2+ = 3d8.
In paramagnetic state, the hybridisation is sp3 and geometry is tetrahedral.
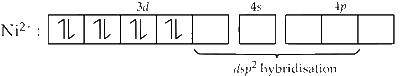
In diamagnetic state, the hybridisation is dsp2 and geometry is square planar.
Comments (0)


