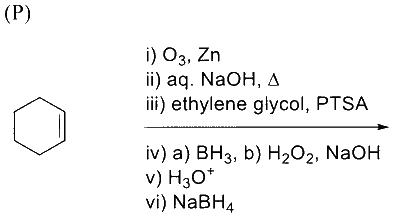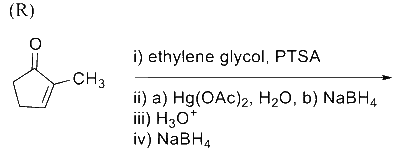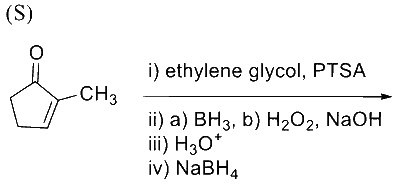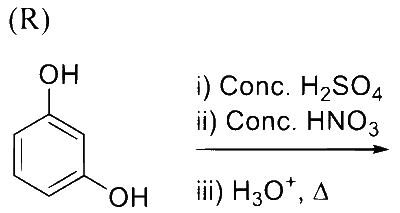JEE Advance - Chemistry (2024 - Paper 1 Online)
Among the following options, select the option in which each complex in Set-I shows geometrical isomerism and the two complexes in Set-II are ionization isomers of each other.
$$ \text { [en }=\mathrm{H}_2 \mathrm{NCH}_2 \mathrm{CH}_2 \mathrm{NH}_2 \text { ] } $$
Set-I: $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3\left(\mathrm{NO}_2\right)_3\right]$ and $\left[\mathrm{Co}(\mathrm{en})_2 \mathrm{Cl}_2\right]$
Set-II: $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{SO}_4$ and $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{SO}_4\right)\right] \mathrm{Cl}$
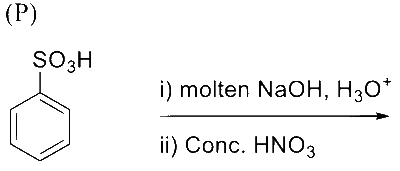
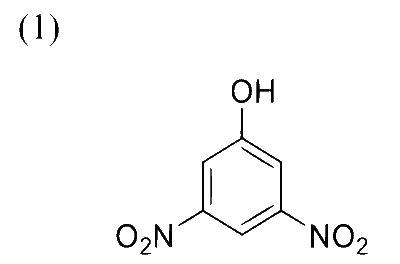
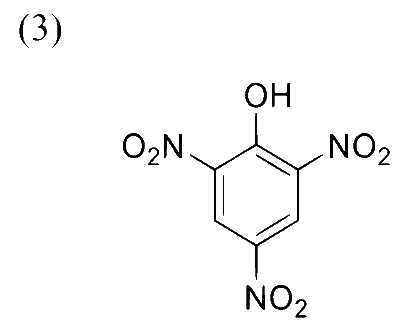
Reaction of iso-propylbenzene with $\mathrm{O}_2$ followed by the treatment with $\mathrm{H}_3 \mathrm{O}^{+}$forms phenol and a by-product $\mathbf{P}$. Reaction of $\mathbf{P}$ with 3 equivalents of $\mathrm{Cl}_2$ gives compound $\mathbf{Q}$. Treatment of $\mathbf{Q}$ with $\mathrm{Ca}(\mathrm{OH})_2$ produces compound $\mathbf{R}$ and calcium salt $\mathbf{S}$.
The correct statement(s) regarding $\mathbf{P}, \mathbf{Q}, \mathbf{R}$ and $\mathbf{S}$ is(are)
Consider the following volume-temperature $(\mathrm{V}-\mathrm{T})$ diagram for the expansion of 5 moles of an ideal monoatomic gas.
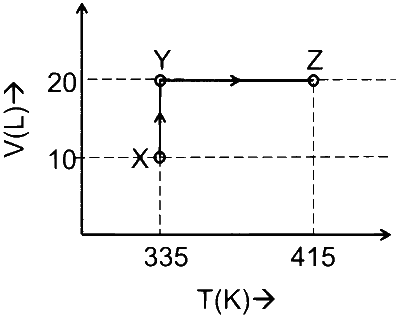
Considering only $\mathrm{P}-\mathrm{V}$ work is involved, the total change in enthalpy (in Joule) for the transformation of state in the sequence $\mathbf{X} \rightarrow \mathbf{Y} \rightarrow \mathbf{Z}$ is ____________.
[Use the given data: Molar heat capacity of the gas for the given temperature range, $\mathrm{C}_{\mathrm{V}, \mathrm{m}}=12 \mathrm{~J} \mathrm{~K}^{-1}$ $\mathrm{mol}^{-1}$ and gas constant, $\left.\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right]$
Consider the following reaction,
$$ 2 \mathrm{H}_2(\mathrm{~g})+2 \mathrm{NO}(\mathrm{g}) \rightarrow \mathrm{N}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) $$
which follows the mechanism given below :
$$ \begin{array}{ll} 2 \mathrm{NO}(\mathrm{g}) \stackrel{k_1}{\underset{k_{-1}}{\rightleftharpoons}} \mathrm{N}_2 \mathrm{O}_2(\mathrm{~g}) & \text { (fast equlibrium) } \\\\ \mathrm{N}_2 \mathrm{O}_2(\mathrm{~g})+\mathrm{H}_2(\mathrm{~g}) \xrightarrow{k_2} \mathrm{~N}_2 \mathrm{O}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) & \text { (slow reaction) } \\\\ \mathrm{N}_2 \mathrm{O}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g}) \xrightarrow{k_3} \mathrm{~N}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) & \text { (fast reaction) } \end{array} $$
The order of the reaction is __________.
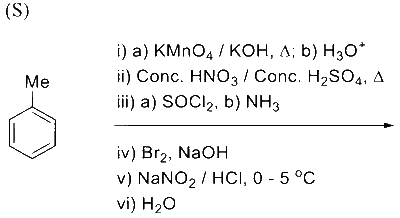
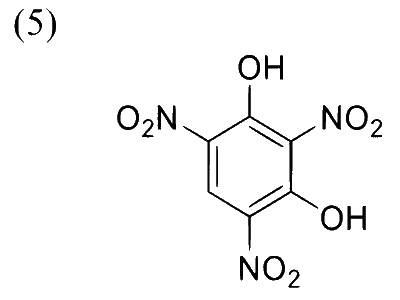
Complete reaction of acetaldehyde with excess formaldehyde, upon heating with conc. $\mathrm{NaOH}$ solution, gives $\mathbf{P}$ and $\mathbf{Q}$. Compound $\mathbf{P}$ does not give Tollens' test, whereas $\mathbf{Q}$ on acidification gives positive Tollens' test. Treatment of $\mathbf{P}$ with excess cyclohexanone in the presence of catalytic amount of $p$-toluenesulfonic acid (PTSA) gives product $\mathbf{R}$.
Sum of the number of methylene groups $\left(-\mathrm{CH}_2-\right)$ and oxygen atoms in $\mathbf{R}$ is __________.
Among $\mathrm{V}(\mathrm{CO})_6, \mathrm{Cr}(\mathrm{CO})_5, \mathrm{Cu}(\mathrm{CO})_3, \mathrm{Mn}(\mathrm{CO})_5, \mathrm{Fe}(\mathrm{CO})_5,\left[\mathrm{Co}(\mathrm{CO})_3\right]^{3-},\left[\mathrm{Cr}(\mathrm{CO})_4\right]^{4-}$, and $\operatorname{Ir}(\mathrm{CO})_3$, the total number of species isoelectronic with $\mathrm{Ni}(\mathrm{CO})_4$ is _________.
[Given, atomic number: $\mathrm{V}=23, \mathrm{Cr}=24, \mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27, \mathrm{Ni}=28, \mathrm{Cu}=29, \mathrm{Ir}=77$ ]
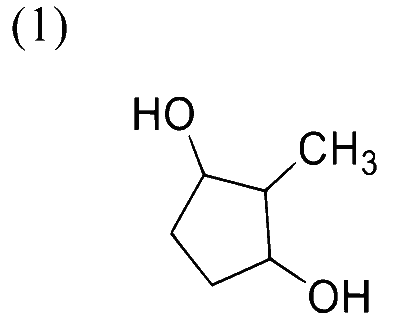
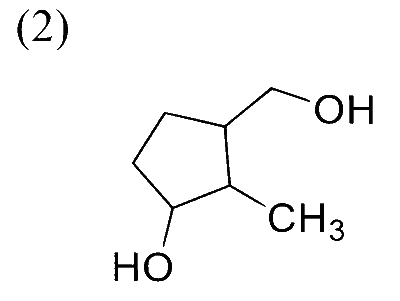
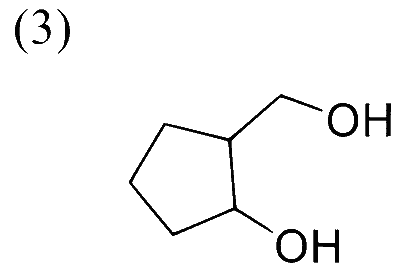
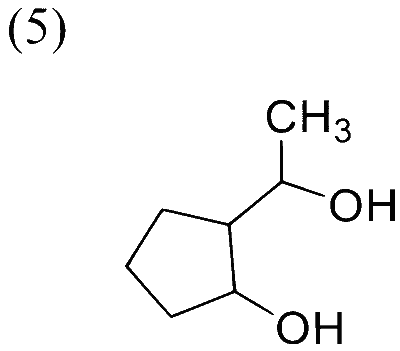
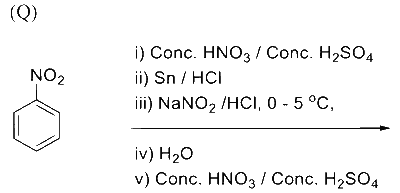
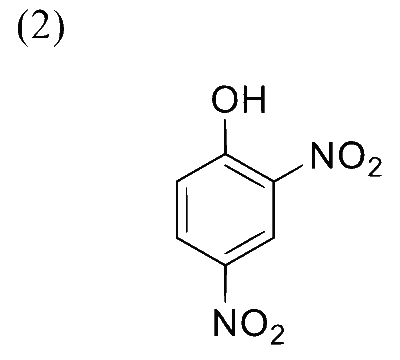
In the following reaction sequence, the major product $\mathbf{P}$ is formed.

Glycerol reacts completely with excess $\mathbf{P}$ in the presence of an acid catalyst to form $\mathbf{Q}$. Reaction of $\mathbf{Q}$ with excess $\mathrm{NaOH}$ followed by the treatment with $\mathrm{CaCl}_2$ yields Ca-soap $\mathbf{R}$, quantitatively. Starting with one mole of $\mathbf{Q}$, the amount of $\mathbf{R}$ produced in gram is __________.
[Given, atomic weight: $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{Na}=23, \mathrm{Cl}=35, \mathrm{Ca}=40$ ]
Among the following complexes, the total number of diamagnetic species is ___________.
$\left[\mathrm{Mn}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{MnCl}_6\right]^{3-},\left[\mathrm{FeF}_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Fe}\left(\mathrm{NH}_3\right)_6\right]^{3+}$, and $\left[\mathrm{Co}(\mathrm{en})_3\right]^{3+}$
[Given, atomic number: $\mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27$;
$$ \text { en } \left.=\mathrm{H}_2 \mathrm{NCH}_2 \mathrm{CH}_2 \mathrm{NH}_2\right] $$
In a conductometric titration, small volume of titrant of higher concentration is added stepwise to a larger volume of titrate of much lower concentration, and the conductance is measured after each addition.
The limiting ionic conductivity $\left(\Lambda_0\right)$ values (in $\mathrm{mS} \mathrm{m}{ }^2 \mathrm{~mol}^{-1}$ ) for different ions in aqueous solutions are given below:
$$ \begin{array}{|c|c|c|c|c|c|c|c|c|c|} \hline \text { Ions } & \mathrm{Ag}^{+} & \mathrm{K}^{+} & \mathrm{Na}^{+} & \mathrm{H}^{+} & \mathrm{NO}_3^{-} & \mathrm{Cl}^{-} & \mathrm{SO}_4^{2-} & \mathrm{OH}^{-} & \mathrm{CH}_3 \mathrm{COO}^{-} \\ \hline \Lambda_0 & 6.2 & 7.4 & 5.0 & 35.0 & 7.2 & 7.6 & 16.0 & 19.9 & 4.1 \\ \hline \end{array} $$
For different combinations of titrates and titrants given in List-I, the graphs of 'conductance' versus 'volume of titrant' are given in List-II.
Match each entry in List-I with the appropriate entry in List-II and choose the correct option.
| LIST-I | LIST-II |
|---|---|
| (P) Titrate: KCl Titrant: AgNO$_3$ |
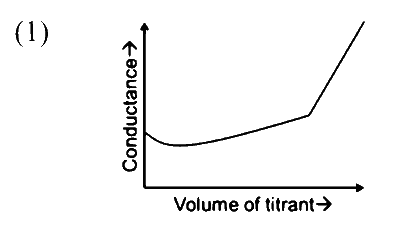
|
| (Q) Titrate: AgNO$_3$ Titrant: KCl |

|
| (R) Titrate: NaOH Titrant: HCl |
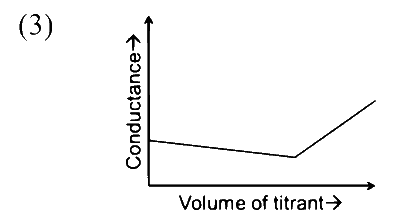
|
| (S) Titrate: NaOH Titrant: CH$_3$COOH |

|

|
Based on VSEPR model, match the xenon compounds given in List-I with the corresponding geometries and the number of lone pairs on xenon given in List-II and choose the correct option.
| List-I | List-II |
|---|---|
| (P) XeF$_2$ | (1) Trigonal bipyramidal and two lone pair of electrons |
| (Q) XeF$_4$ | (2) Tetrahedral and one lone pair of electrons |
| (R) XeO$_3$ | (3) Octahedral and two lone pair of electrons |
| (S) XeO$_3$F$_2$ | (4) Trigonal bipyramidal and no lone pair of electrons |
| (5) Trigonal bipyramidal and three lone pair of electrons |