JEE Advance - Chemistry (2024 - Paper 1 Online - No. 8)
Consider the following volume-temperature $(\mathrm{V}-\mathrm{T})$ diagram for the expansion of 5 moles of an ideal monoatomic gas.
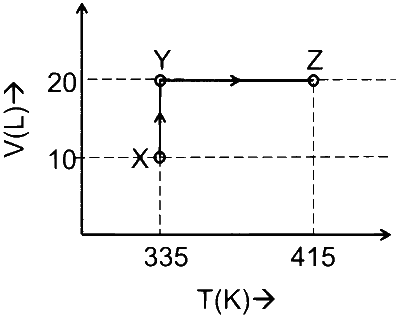
Considering only $\mathrm{P}-\mathrm{V}$ work is involved, the total change in enthalpy (in Joule) for the transformation of state in the sequence $\mathbf{X} \rightarrow \mathbf{Y} \rightarrow \mathbf{Z}$ is ____________.
[Use the given data: Molar heat capacity of the gas for the given temperature range, $\mathrm{C}_{\mathrm{V}, \mathrm{m}}=12 \mathrm{~J} \mathrm{~K}^{-1}$ $\mathrm{mol}^{-1}$ and gas constant, $\left.\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right]$
Explanation
For ideal gas
$$ \begin{aligned} & \Delta H=\mathrm{nC}_{\mathrm{P}} \Delta \mathrm{T} \\\\ & \because \mathrm{C}_{\mathrm{P}}=\mathrm{C}_{\mathrm{V}}+\mathrm{R}=12+8.3=20.3 \mathrm{~J} / \mathrm{K} \text {-mole } \\\\ & \therefore \Delta \mathrm{H}=5 \times 20.3 \times(415-335) \\\\ & \Delta \mathrm{H}=8120 \text { Joule } \end{aligned} $$
Comments (0)


