JEE Advance - Chemistry (2024 - Paper 1 Online - No. 4)
Among the following options, select the option in which each complex in Set-I shows geometrical isomerism and the two complexes in Set-II are ionization isomers of each other.
$$ \text { [en }=\mathrm{H}_2 \mathrm{NCH}_2 \mathrm{CH}_2 \mathrm{NH}_2 \text { ] } $$
Set-I: $\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ and $\left[\mathrm{PdCl}_2\left(\mathrm{PPh}_3\right)_2\right]$
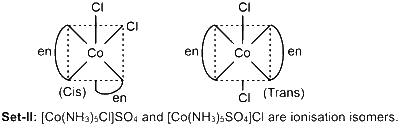
Set-II: $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{SO}_4$ and $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{SO}_4\right)\right] \mathrm{Cl}$
Set-I: $\left[\mathrm{Co}(\mathrm{en})\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]$ and $\left[\mathrm{PdCl}_2\left(\mathrm{PPh}_3\right)_2\right]$
Set-II: $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]\left[\mathrm{Cr}(\mathrm{CN})_6\right]$ and $\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]\left[\mathrm{Co}(\mathrm{CN})_6\right]$
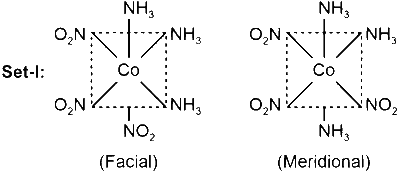
Set-I: $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3\left(\mathrm{NO}_2\right)_3\right]$ and $\left[\mathrm{Co}(\mathrm{en})_2 \mathrm{Cl}_2\right]$
Set-II: $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{SO}_4$ and $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{SO}_4\right)\right] \mathrm{Cl}$
Set-I: $\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{Cl}_2$ and $\left[\mathrm{Co}(\mathrm{en})\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]$
Set-II: $\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_3$ and $\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_5 \mathrm{Cl}\right] \mathrm{Cl}_2 \cdot \mathrm{H}_2 \mathrm{O}$
Explanation
Comments (0)


