JEE Advance - Chemistry (2024 - Paper 1 Online - No. 15)
Based on VSEPR model, match the xenon compounds given in List-I with the corresponding geometries and the number of lone pairs on xenon given in List-II and choose the correct option.
| List-I | List-II |
|---|---|
| (P) XeF$_2$ | (1) Trigonal bipyramidal and two lone pair of electrons |
| (Q) XeF$_4$ | (2) Tetrahedral and one lone pair of electrons |
| (R) XeO$_3$ | (3) Octahedral and two lone pair of electrons |
| (S) XeO$_3$F$_2$ | (4) Trigonal bipyramidal and no lone pair of electrons |
| (5) Trigonal bipyramidal and three lone pair of electrons |
Explanation
$\mathrm{XeF}_2 \Rightarrow 2$ sigma bonds and 3 lone pairs on $\mathrm{Xe}$, number of hybrid orbitals $=5, \mathrm{sp}^3 \mathrm{~d}$ hybridisation , geometry will be trigonal bipyramidal.
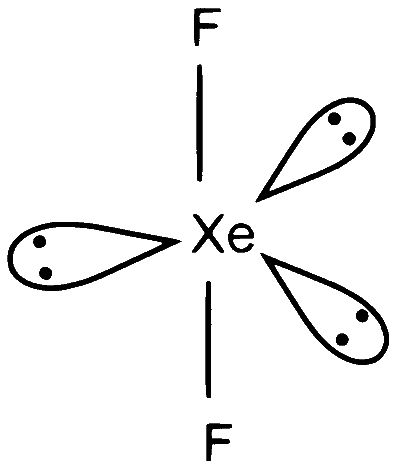
$\mathrm{P}-5$
$\mathrm{XeF}_4 \Rightarrow 4$ sigma bonds and 2 lone pairs on $\mathrm{Xe}$, number of hybrid orbitals $=6, \mathrm{sp}^3 \mathrm{~d}^2$ hybridisation , geometry will be octahedral.
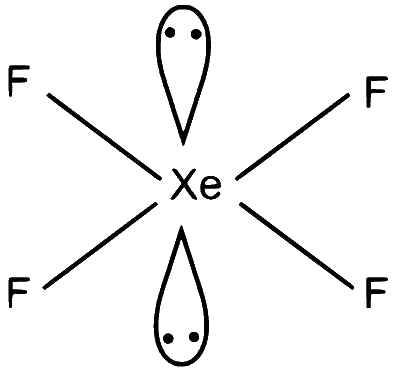
Q-3
$\mathrm{XeO}_3 \Rightarrow 3$ sigma bonds and 1 lone pairs on $\mathrm{Xe}$, number of hybrid orbitals $=4, \mathrm{sp}^3$ hybridisation , geometry will be tetrahedral.
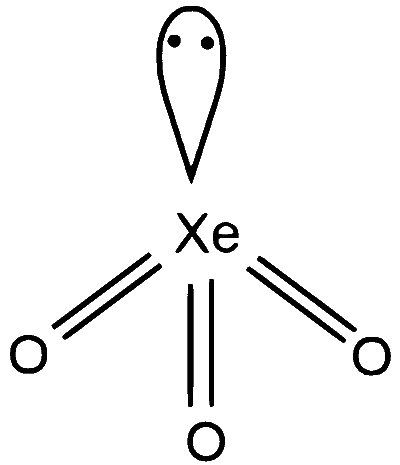
R-2
$\mathrm{XeO}_3 \mathrm{F}_2 \Rightarrow 5$ sigma bonds and 0 lone pairs on $\mathrm{Xe}$, number of hybrid orbitals $=5, \mathrm{sp}^3 \mathrm{~d}$ hybridisation , geometry will be trigonal bipyramidal.
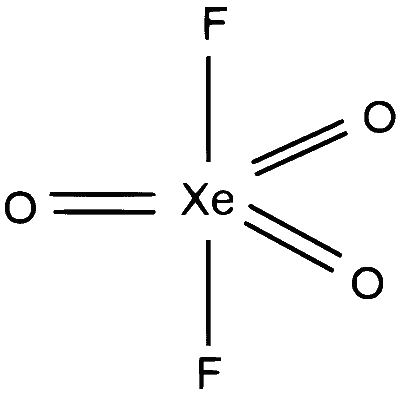
S-4
Comments (0)


